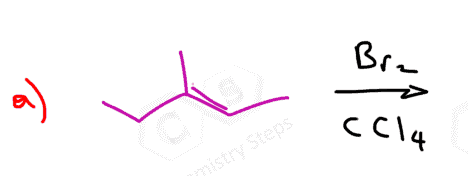
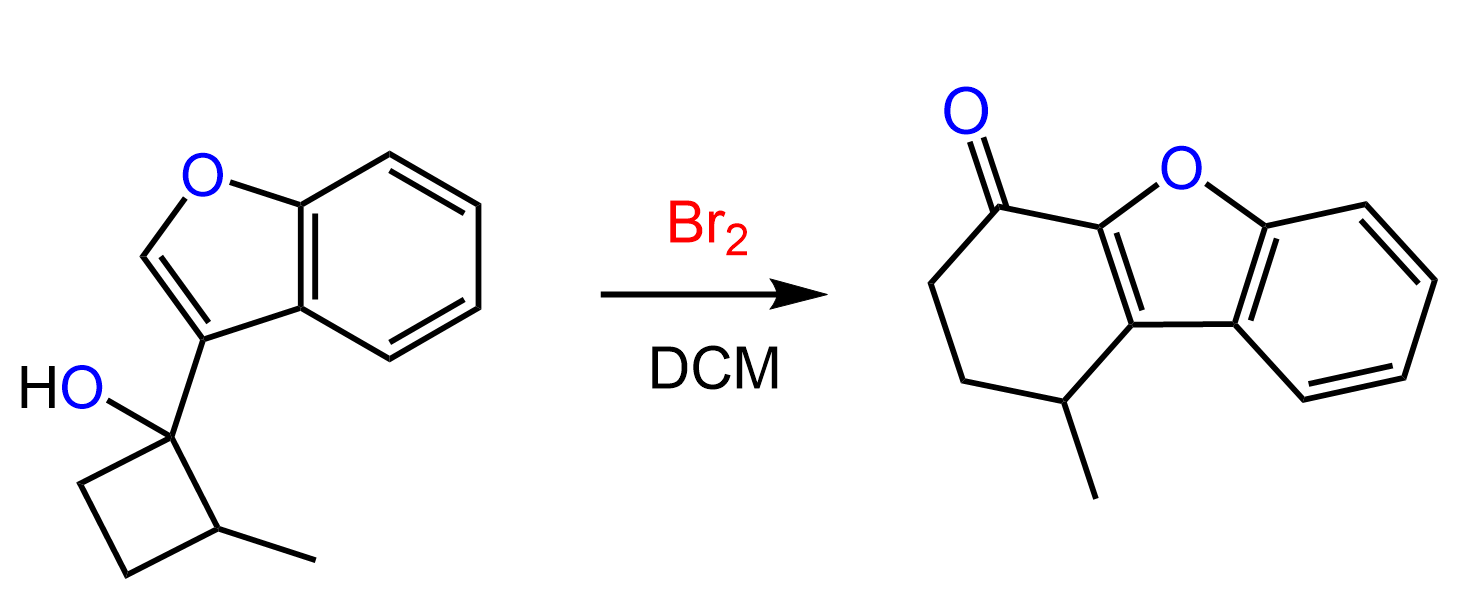
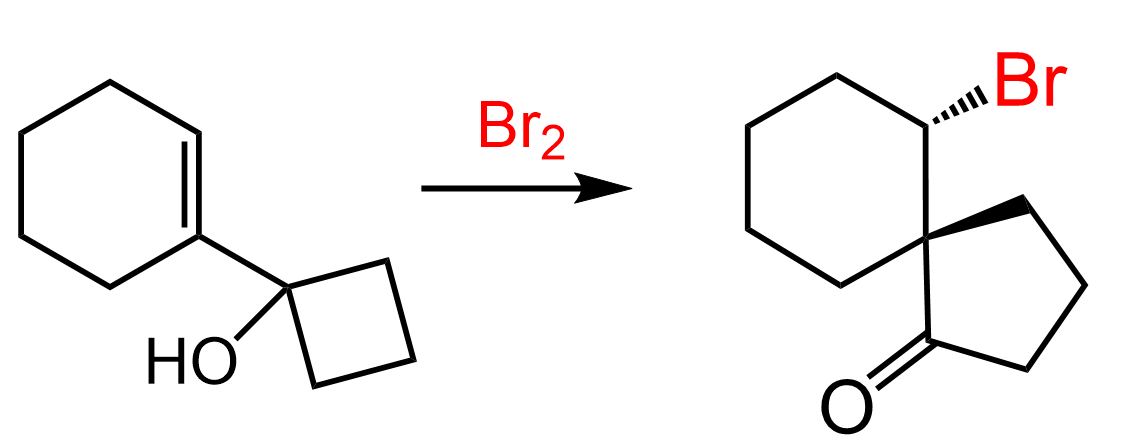
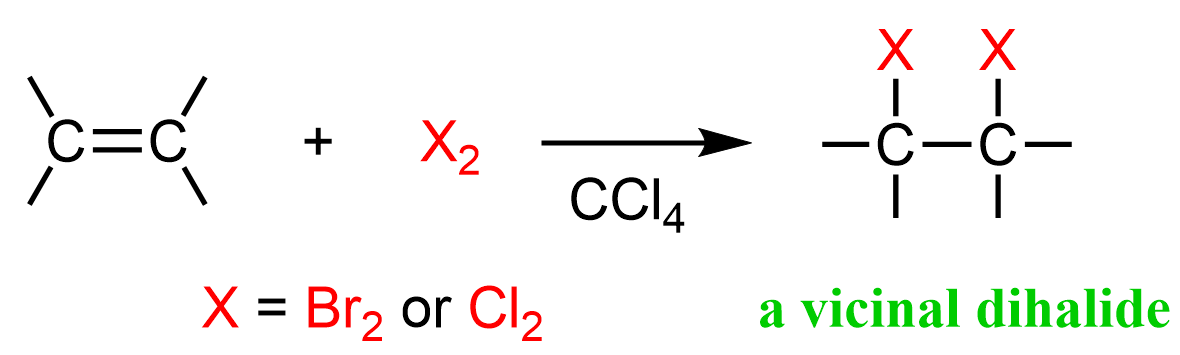
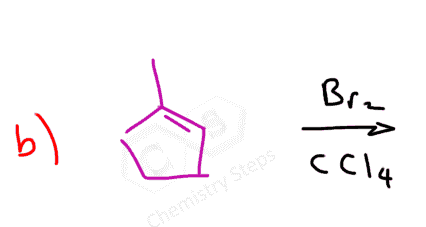The halogens Br2 and Cl2 add to the double bond of an alkene producing vicinal dihalides – a compound bearing the halogens on adjacent carbons (vicinus, Latin: adjacent). These are also called 1,2-dihalides:
The reaction with bromine is a standard test for the presence of a π bond. Bromine is dark red liquid and upon reacting with a double bond, turns colorless.
Mixed halogenations can also be achieved. For example, a mixture of Br2 and Cl2 have been used to perform bromochlorination.
The reaction is possible because the halogen bond is relatively weak and polarizable. When the electron-rich π bond approaches the halogen, it causes one of the atoms to have a partial positive charge and it now becomes the electrophile that the alkene attacks:

Mechanism and Stereochemistry
One may expect this to be similar to the hydrogen halide addition mechanism where the protonation of the double produces a carbocation according to the Markovnikov’s rule:

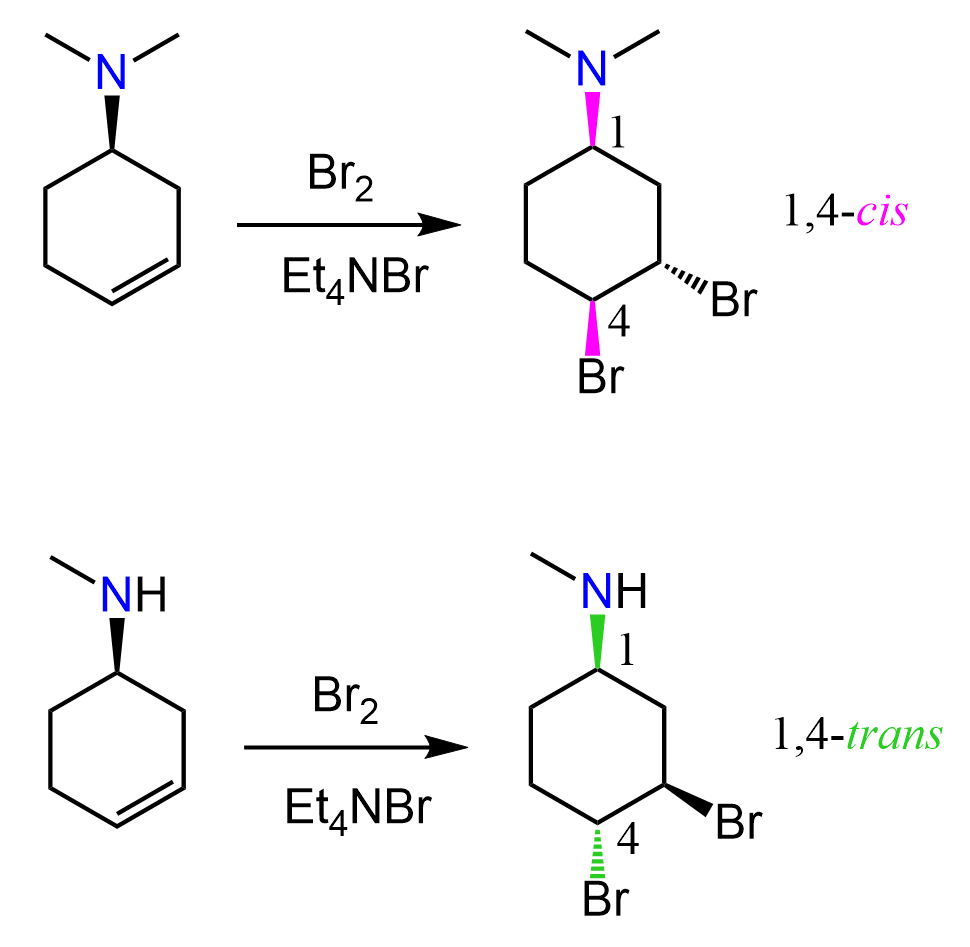
This mechanism, however, does not explain the exclusive anti-addition of the halogen. For example, the addition of bromine to cyclohexene produces trans-1,2-dibromocyclohexane, and cis-1,2-dibromocyclopentane is not observed:

The mechanism that explains this stereochemistry involves a cyclic bromonium ion intermediate.
The p electrons of the π bond attack the Br2 to make a new σ bond with it and the other bromine leaves with the electron pair. This, however, does not form a carbocation since the electron cloud of the bonded bromine is very close to the other sp2 carbon and forms a new bond with it. As a result, instead of the common carbocation in the addition reactions, a cyclic bromonium ion intermediate is formed:
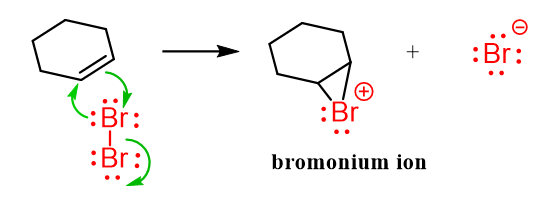
An important thing to mention here is that because there is no carbocation formed, the halogenation and other reactions with the halonium ions do not involve rearrangements.
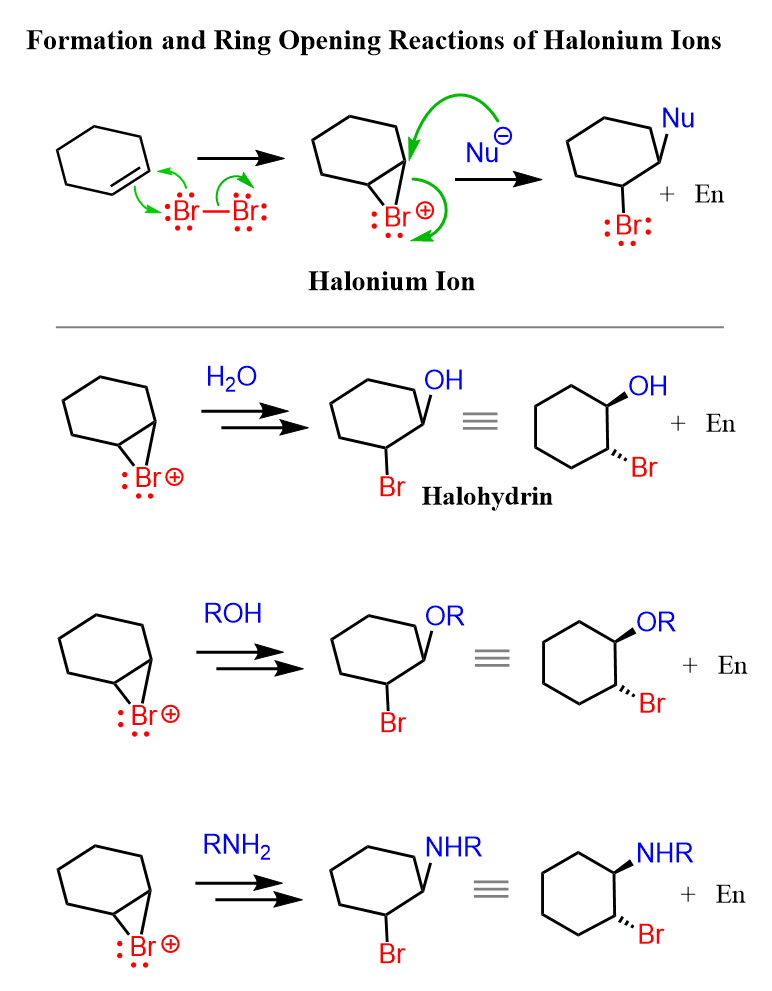
This cyclic intermediate is a three-membered ring that is unstable because of the high ring strain and is susceptible to nucleophilic attacks as we have also seen in the oxymercuration reaction. In addition, the bromine is positively charged which makes it an excellent leaving group in a nucleophilic substitution reaction.
The question is, who is the nucleophile? And this is what the Br– that was expelled during the ring formation does. It attacks the carbon by SN2 mechanism, releasing the strain and forming the final dihalide:

This dihalide is a chiral compound, however, it forms as a racemic mixture. The initial addition of the Br to the alkene occurs from both faces of the double bond forming two enantiomers of the bromonium ion. The consequent attack of the Br– produces both enantiomers in equal amounts:

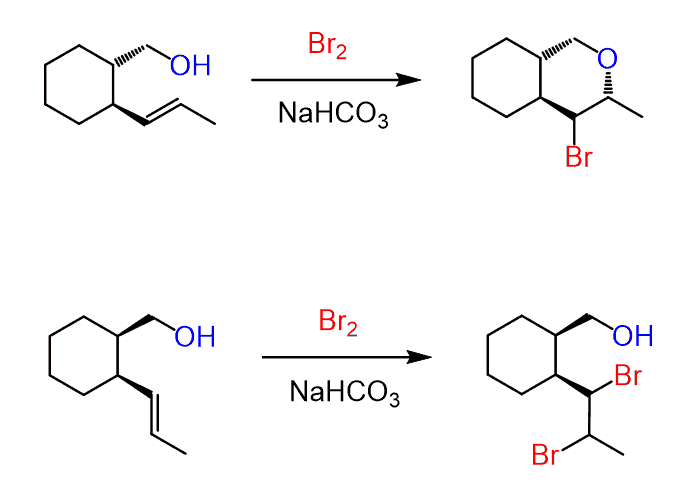
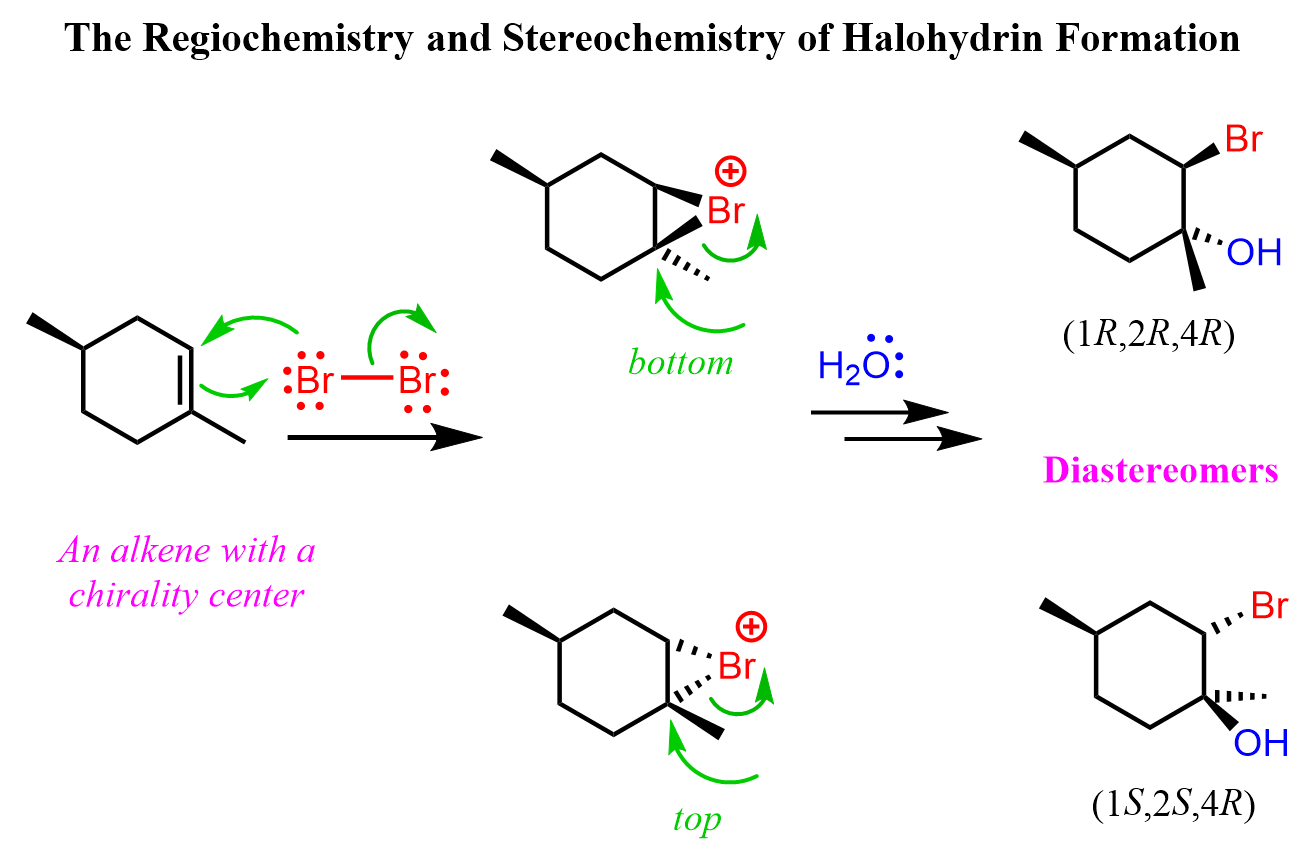
If the alkene contains a chirality center, then a pair of diastereomers are formed because while the newly-forming centers are going to be R and S, the original chirality center is intact, thus the halohydrins are not mirror images:
Watch out for meso compounds. Not every dihalide with stereogenic centers is going to be chiral:

Chlorine reacts the same way with alkenes forming chloronium ion, which in general is called halonium ion.
F2 and I2 are not synthetically useful for this reaction as F2 reacts explosively with the alkene while the reaction with I2 does not proceed to a significant extent:

The halogenation of alkenes is carried out in a neutral organic solvent such as carbon tetrachloride (CCl4 or dichloromethane, DCM (CH2Cl2) that cannot act as a nucleophile when the halonium ion is formed.
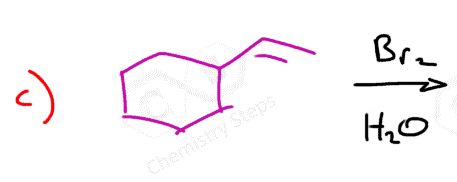
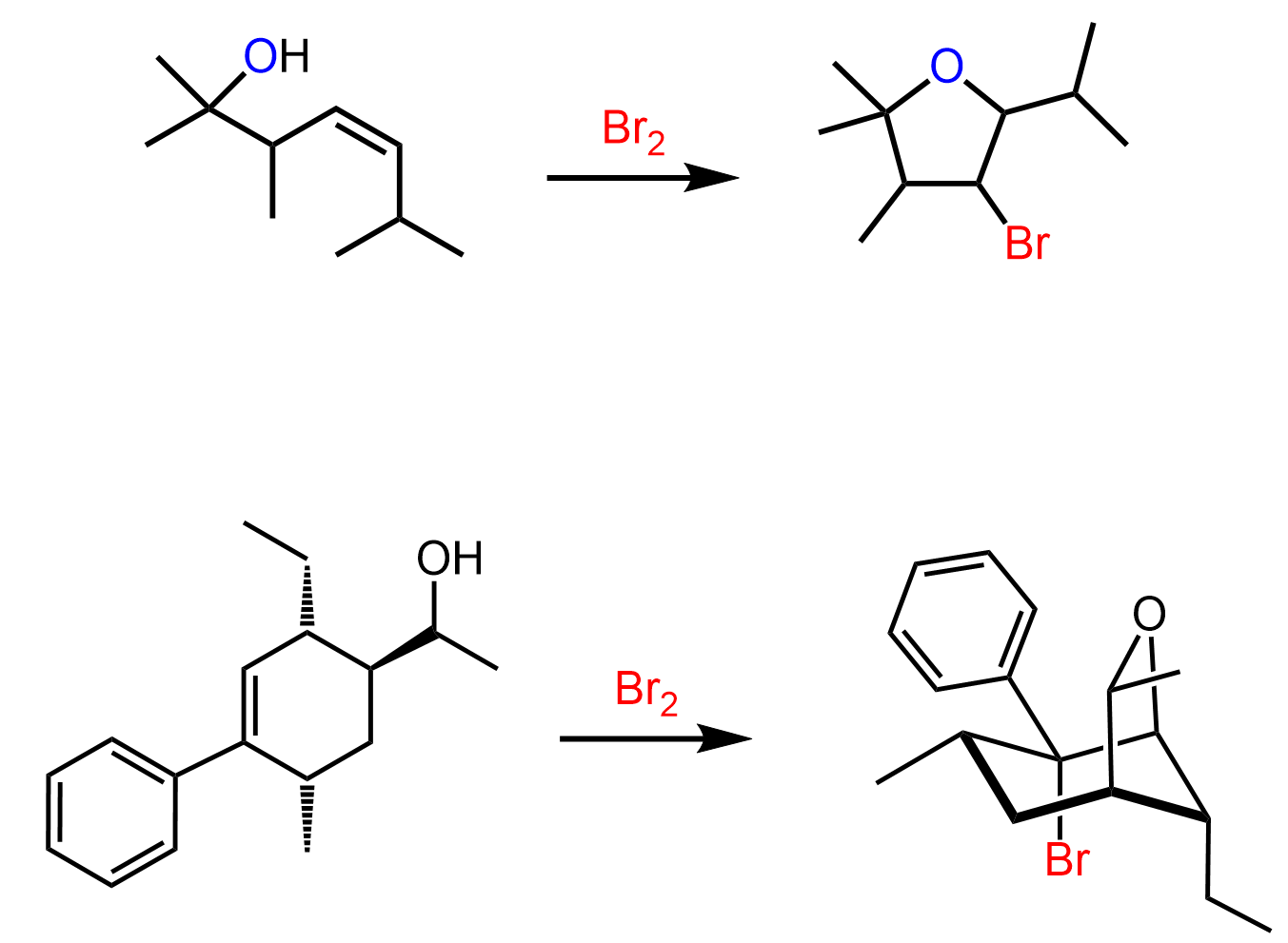
On the other hand, if the reaction is carried out in water, for example, a halohydrin is formed by the addition of the water to the halonium ion:

Even though the Br– or Cl– are close to performing the nucleophilic attack, water has the advantage of being in huge excess as it is often used as the solvent.
It still follows the same mechanism; therefore, the anti-addition occurs and trans products are formed:
Regiochemistry
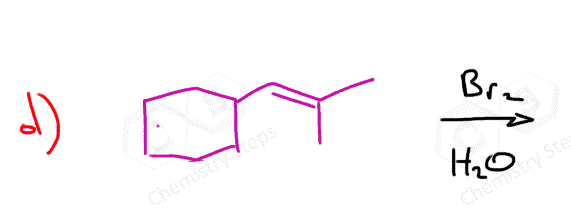
When an unsymmetrical alkene is used, the resulting halonium ion can be attacked by a nucleophile at the two carbon atoms connected to the halogen:

And it turns out that the nucleophile attacks the more substituted carbon atom:

This is explained by the stability of the energy differences between the two possible transition states:
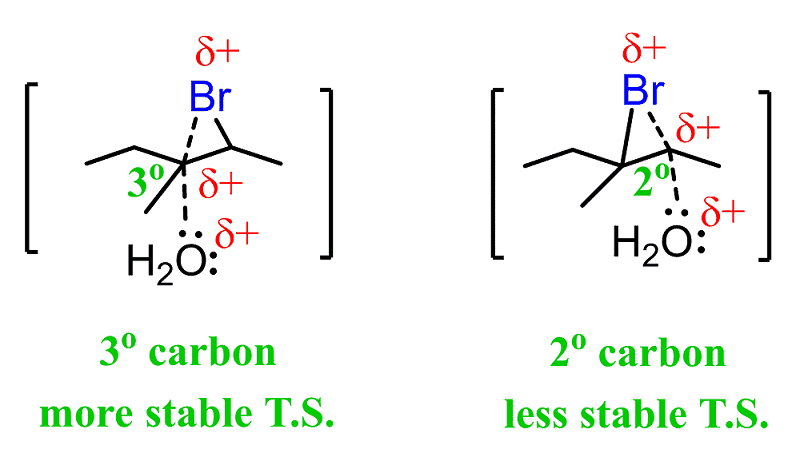
The first transition state has a partial positive charge on a more substituted carbon, making it more stable.
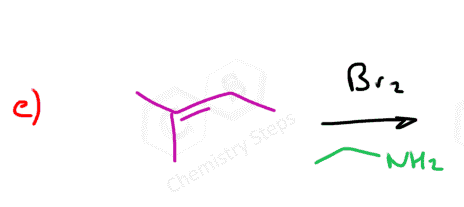
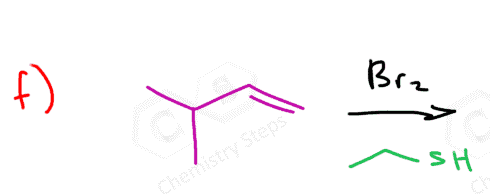
When other nucleophiles such as alcohols, amines, thiols, etc. are added to the reaction mixture they react with the halonium ion in the same way.