Alkynes
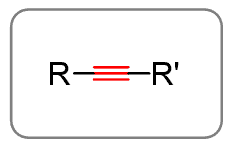
Alkynes are unsaturated hydrocarbons that contain a carbon–carbon triple bond. Alkynes are unsaturated because they have fewer hydrogen atoms than the corresponding alkenes and therefore the general formula is CnH2n-2. Remember, the general formula for alkanes is CnH2n+2. That is a four-hydrogen difference, which corresponds to two degrees of unsaturation.

In the nomenclature of alkynes, the ending changes from “ane” to “yne”:

Alkynes are classified into two main types – Internal and Terminal Alkynes.
If the triple bond is at the periphery, the alkyne is classified as terminal. These are also known as monosubstituted acetylenes.
In disubstituted acetylenes, the triple bond is located elsewhere along the chain:

Structure and Hybridization of Alkynes
The carbon atoms in the triple bond are sp-hybridized. Remember, in the sp hybridization, the s orbital of the excited state carbon is mixed with only one out of the three 2p orbitals. It is called sp hybridization because two orbitals (one s and one p) are mixed:

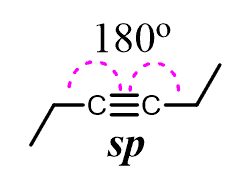
The resulting two sp hybrid orbitals are then arranged in a linear geometry (180o), and the two unhybridized 2p orbitals are placed at 90o:

Let’s see how this happens in acetylene- C2H2. The two carbon atoms make a sigma bond by overlapping the sp orbitals.

One hydrogen bonds to each carbon atom by overlapping its s orbital with the other sp orbital.
The two p orbitals of each carbon overlap to make two π bonds.
The key parameters of the sp hybridization and triple bond:
* In a triple bond, there is one σ (sigma) and two π (pi) bonds.
* All the atoms have linear geometry.
* The angle between atoms is 180o which corresponds to the linear geometry:
This angle occasionally may vary since alkynes can exist as cyclic compounds. The smallest cycloalkyne is a 9-membered ring cyclononyne.
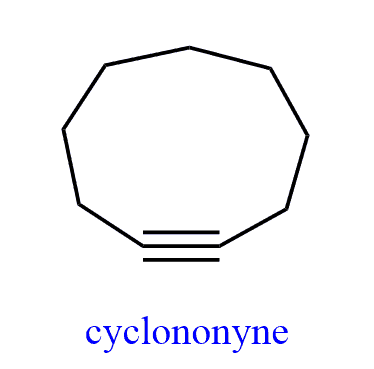
However, due to the ring strain, it decomposes at room temperature.
The Acidity of Terminal Alkynes
Alkynes are a lot more acidic than alkanes and alkenes:
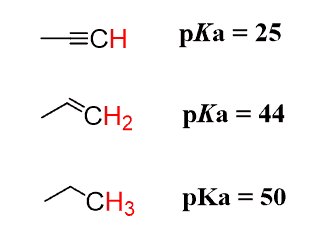
This trend cannot be explained by any of the factors of “Atom”, “Resonance”, and “Induction” discussed in ARIO. We have the same atom (carbon), no resonance stabilization, and no inductive effect.
The last factor to consider is the orbital. Let’s put a negative charge on each carbon and take a closer look:

The only difference in these carbons is their hybridization state. Remember, alkanes are sp3, alkenes are sp2, and alkynes are sp-hybridized.
What you need to know is that s orbitals are more electronegative than p orbitals, and the more s character the hybrid orbital has, the better it stabilizes the negative charge. These are the percentages of the s orbital (s-character) in each hybrid orbital:

This is an important feature that makes it possible to deprotonate terminal alkynes with strong yet safe-to-handle bases such as the sodium amide or sodium hydride:

The resulting acetylide ion is both a strong base and an excellent nucleophile, which is why it is a very important reagent in organic synthesis.
When reacted with sterically unhindered 1o substrates, they give nucleophilic substitution by the SN2 mechanism:
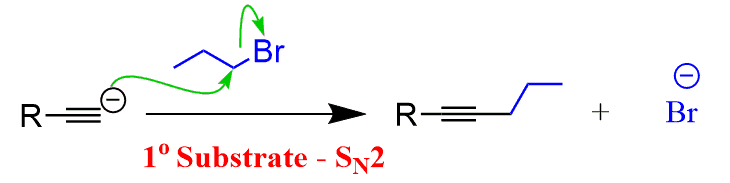
More details and practice problems can be found at Alkylation of Terminal Alkynes in Organic Synthesis
Organic Chemistry Final Practice Quiz
Alkyne Naming and Reactions Practice Quiz
Check Also
- Naming Alkynes by IUPAC Nomenclature Rules – Practice Problems
- Preparation of Alkynes by Elimination Reactions
- Hydrohalogenation of Alkynes
- Addition of Water to Alkynes
- Acid-Catalyzed Hydration of Alkynes with Practice Problems
- Reduction of Alkynes
- Halogenation of Alkynes
- Hydroboration-Oxidation of Alkynes with Practice Problems
- Ozonolysis of Alkynes with Practice Problems
- Alkylation of Terminal Alkynes in Organic Synthesis with Practice Problems
- Reactions of Acetylide Ions
- Alkyne reactions summary practice problems
- Alkyne Synthesis Reactions Practice Problems
- Alkyne Naming and Reactions Practice Quiz
- Reactions Map of Alkynes


Hello will the info for introduction to Alkynes be posted soon?
May you please post the quiz set for the Alkyne reaction practice problems?
Currently working on the quiz when I get time outside of classes. No timeline yet as it does involve a lot of work and it is the final’s season:) You can use the practice problems after the articles.
I second this. The alkene quiz was very helpful. Please add one for alkynes.
Where do I find this quiz?
The Alkyne quiz has been added, currently with 43 questions. I am working on bringing the number to 60-70.
The link to the quiz is above, after the article.
Thank you! The quizzes are helpful especially with the hints and study guides.
Thank youuuuu so much!