Esters can be converted to alcohols by two types of reduction reactions. One is the reduction by a strong reducing agent such as LiAlH4 to a primary alcohol, and the second is the conversion to a tertiary alcohol by reacting with two equivalents of Grignard or organolithium reagent.
Ester Reduction with LiAlH4
The reduction of an ester to an alcohol requires two hydride additions to the carbonyl group and therefore an excess of LiAlH4 is used:

This is because the tetrahedral intermediate formed after the first hydride addition contains a leaving group which is kicked out re-forming the carbonyl group:

The newly formed carbonyl group is an aldehyde and it is more reactive than the ester, thus is attacked one more time by LiAlH4.

Sometimes, this reduction can also be achieved with DIBAL-H. You can read more about the mechanisms and selectivity of reducing carbonyl compounds here.
Esters with Grignard and Organolithium
Esters can be converted to tertiary alcohols by reacting them with two equivalents of a Grignard reagent:
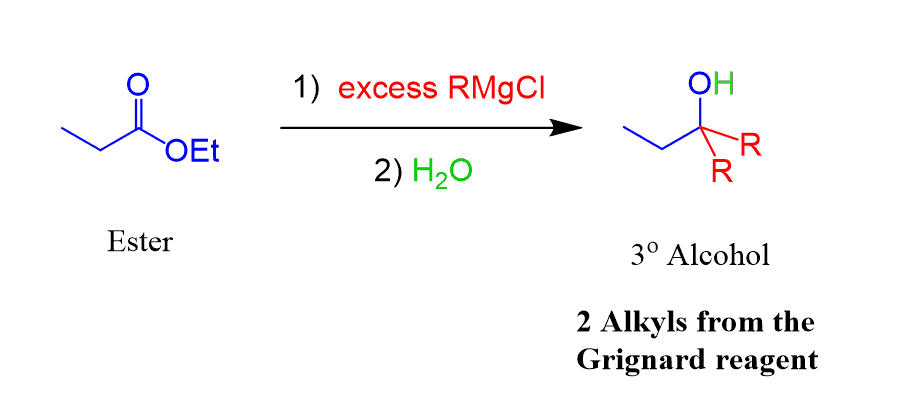
In the first step, we have the nucleophilic attack of the Grignard, making the C-C bond and shifting the electrons of the π bond to the oxygen. The second equivalent is needed because the product of the first addition-elimination reaction to the carbonyl is a ketone (was an aldehyde in the case of LiAlH4), which is now converted into a tertiary alcohol:

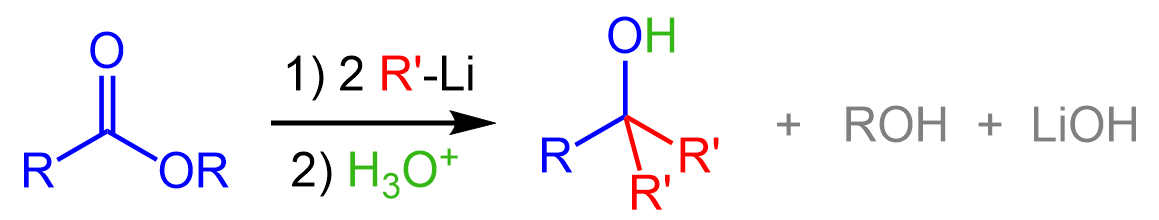
In a similar way, organolithiums will also convert an ester to a tertiary alcohol:
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Reduction of Carbonyl Compounds by Hydride Ion
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems

