Let’s first define the meaning of a strong acid. According to the Arrhenius theory, acids dissociate in water to form protons (H+). The general equation for acid dissociation can be shown as:

For a specific example, we can look at the dissociation of hydrochloric acid:
![]()
In this case, the conjugate base of the acid is the chloride ion. Hydrochloric acid is a strong acid because it completely ionizes in aqueous solutions.
So, one measure of acid strength is the extent of its dissociation, which indicates the amount of protons or hydronium ions that it produces in the solution.
The more the acid dissociates into a proton, the stronger it is. We can also say, the stronger the tendency of a compound to donate a proton, the stronger acid it is.
The same principle applies to the acidity of organic molecules. However, most organic compounds are weak acids, and their dissociation is shown with a double arrow like we do in equilibrium reactions.
For example, let’s consider the dissociation of ethanol and ethyl amine:
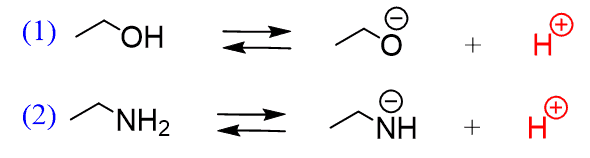
Given equal concentration, whichever produces more H+ is the stronger acid and, it turns out that ethanol is a stronger acid than the ethyl amine because it dissociates to a greater extent than ethyl amine does.
The pKa
Aside from the qualitative comparison of the acid strength, we need a quantitative definition for the acid strength. This is where the pKa comes into play. Acid-dissociation is a reversible reaction described by an equilibrium constant (Keq), which, for the ionization of acids, is denoted as Ka. The pKa is derived from the Ka by taking its negative log. Let’s see how that works:
Before bringing in the numbers, let’s mention that another way of showing the acid-dissociation reaction (which is more accurate) is to include the water and formation of the hydronium ion (H3O+):
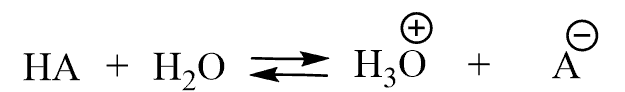
The equilibrium constant for this reaction would be:
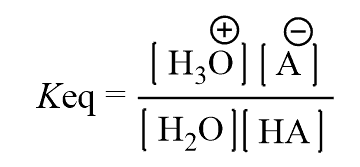
Since the concentration of the water is constant and very large compared to the acid, it is included in the formula of Ka:
![]()
Therefore, the [H2O] term does not appear in the Ka formula, and we have:
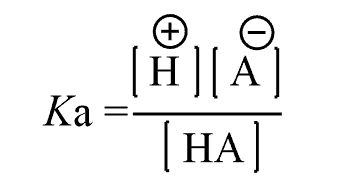
Just like in any equilibrium reaction, the stronger the acid, the larger the Ka.
Now, let’s go back to the dissociation of the alcohol and the ethylamine. The Ka values of ethyl alcohol (1) and ethyl amine (2) are shown below:

The alcohol is a stronger acid because its Ka is higher. To simplify the numbers, -log Ka is used instead of Ka, getting rid of the exponent.
pKa = – logKa
The pKa is the quantitative indicator of the acid strength. For our two compounds, we have
pKa1 = – log10-16 = 16 < pKa2 = – log10-38 = 38
pKa (EtOH) = 16, pKa (EtNH2) = 38
What we notice is that the alcohol is a stronger acid but has a lower pKa value, and this is true for any molecule. Just memorize this!

The following table summarizes the pKa values of most functional groups in organic chemistry:

Download the PDF file of the pKa Table below here, and this is a shorter version of the pKa table as well:

You can use the table to look up the pKa value of a given compound based on the functional group(s) it contains. For example, if you need to find the pKa of isopropanol and it is not in the pKa table, you can go based on the ethanol. Since it is an alcohol and has approximately the same pKa value:

Same for the amines, they all have about the same pKa as ammonia since they are the organic derivatives of ammonia (functional groups).
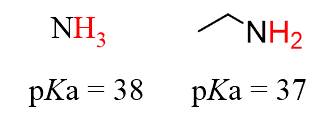
The pKa value does vary for the same functional group in different compounds, but it is within an accepted range of approximation to describe the acid strength and predict acid-base reactions.
Notice also that the pKa scale is logarithmic, so even a small difference, let’s say pKa1 = 16 and pKa2 = 19, represents 3 pKa units, which means the first compound is 103 or 1,000 times more acidic than the second compound. For example:
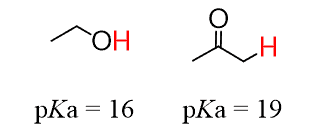
Predicting the Strength of Bases
So far, we have talked about the strength of the acid. However, it is also very important to understand the strength of the base. Let’s look at the dissociation of ethanol and ethylamine one more time:

We have determined that ethanol is a stronger acid, and another way to look at this is to say that the equilibrium is shifted more to the right side (more dissociation) compared to the dissociation of the ethylamine. This, in turn, means that the ethoxide ion is not as reactive as the EtNH– ion because otherwise the equilibrium would have been shifted to the left as much as it is for the amine. So, the ethoxide is more stable, less reactive/active than the EtNH– ion. All of this, in one word, means that it is a weaker base.
This correlation is true for any acid and conjugate base pair:
The stronger the acid, the weaker its conjugate base.
And vice versa:
The weaker the acid, the stronger its conjugate base.
Predicting the More Acidic Compound
If both molecules contain one functional group, then you simply need to look up the pKa values. The one with a lower pKa value is more acidic. For example, which of the following two molecules is more acidic?
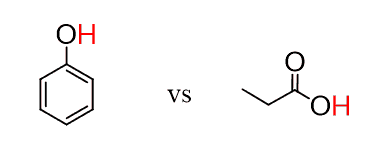
The pKa of carboxylic acids is ~5, and the pKa of phenol is ~10. Therefore, propionic acid is 105 times more acidic than phenol.
What if there are more functional groups in the molecule?
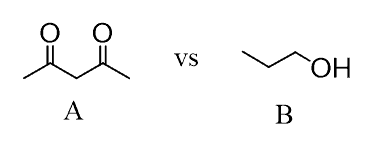
Here, you need to identify the most acidic proton in each molecule. In this case, molecule A is more acidic than molecule B.
How come alcohols aren’t more acidic than ketones?
Yes, they are. However, molecule A has two sets of protons. The ones next to the methyls and the ones in between the two methyl groups, which are a lot more acidic:

This is a dicarbonyl compound, which, because of its unusual acidity, is also called C-H acids.
Look in the table and determine the pKa values for these two protons.
One is ~19, and the other is ~9. That is a huge difference – 1010 times more acidic.
Finally, the pKa of alcohols is about 16, and comparing these values, you can see that molecule A is a million times more acidic than molecule B.
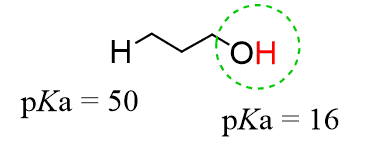
Notice that we did not even look at the pKa values of the other protons in molecule B. These are connected to carbons and range at pKa ~ 50, and obviously, we won’t refer to them for comparing the acidity of alcohols.
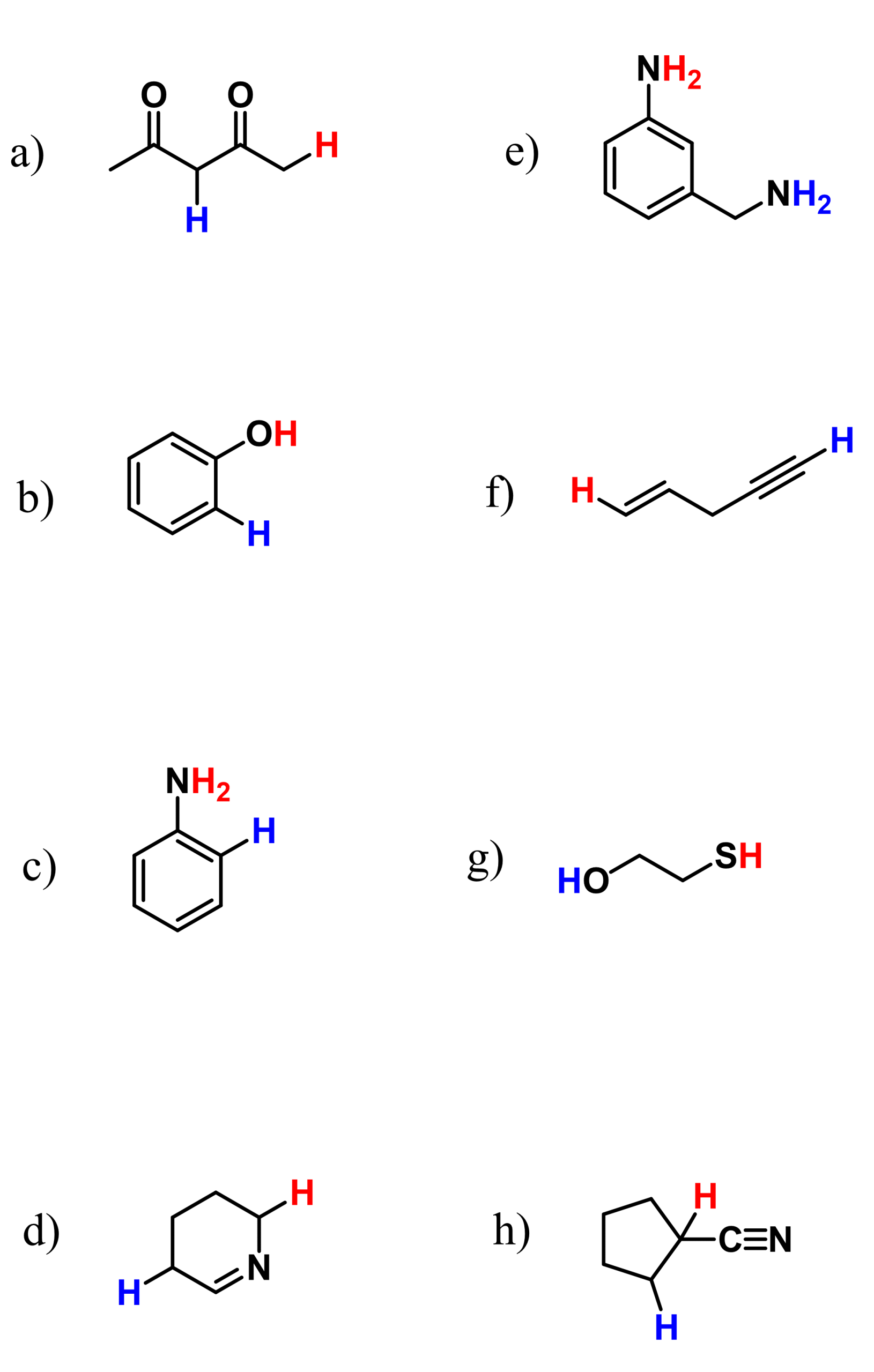
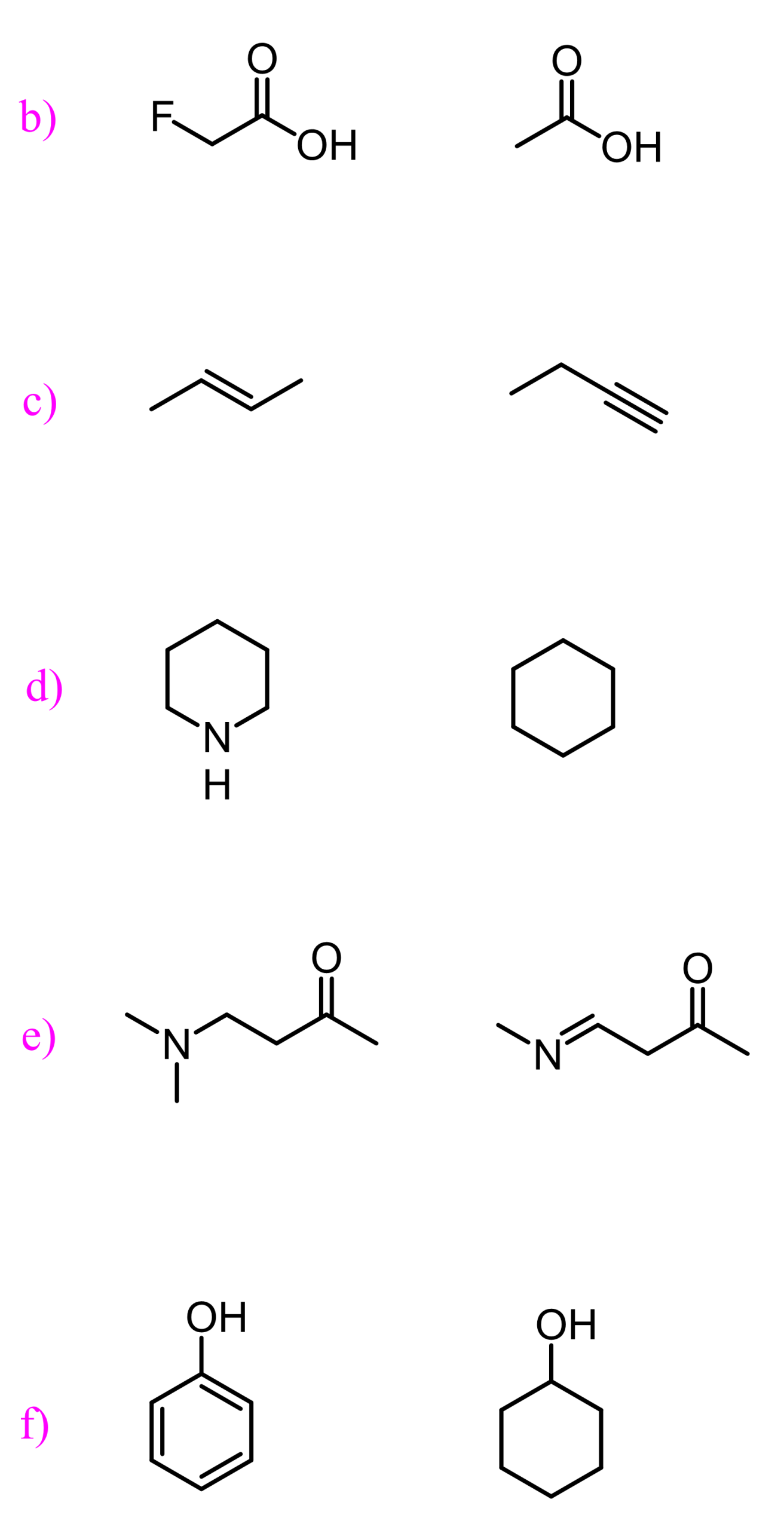
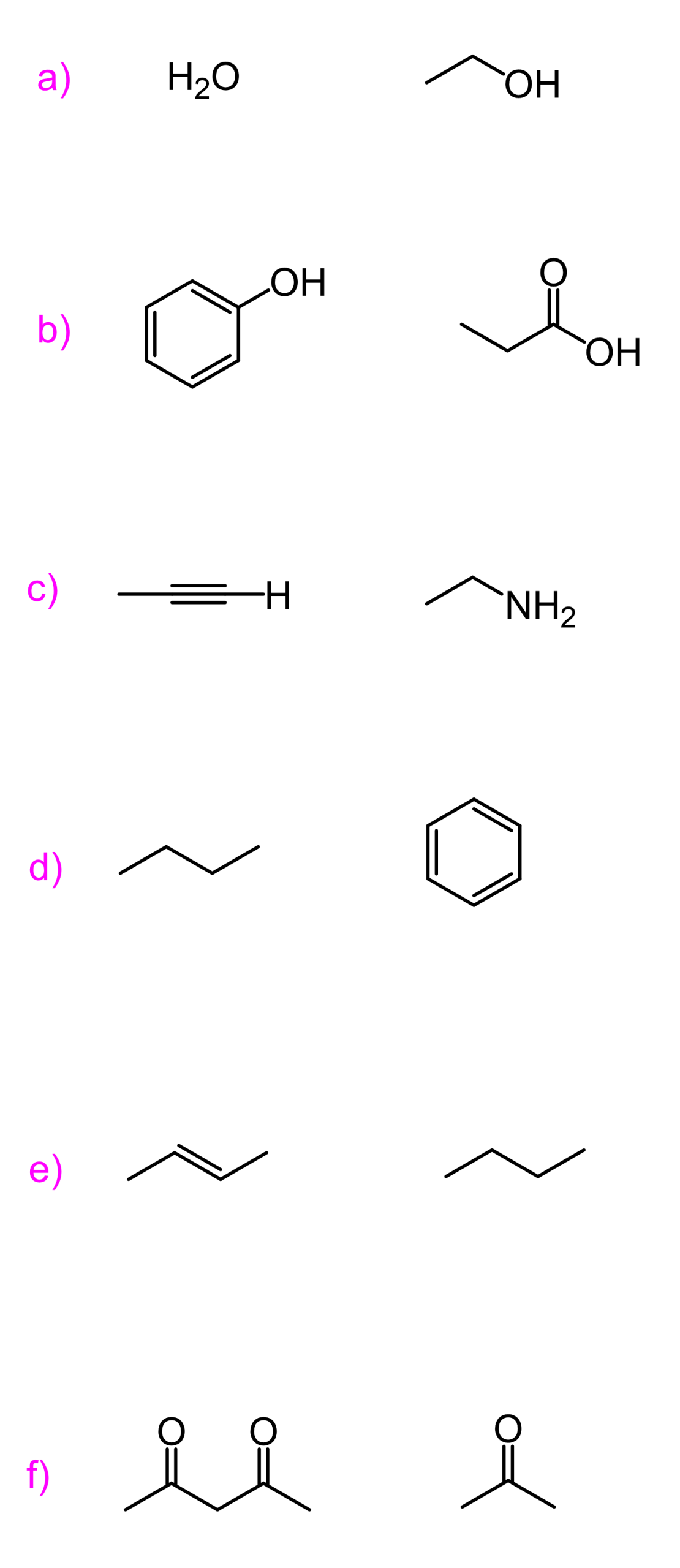
We also have a dedicated post on predicting the most acidic proton in a molecule or a pair of compounds, so check that out as well for more examples and factors affecting acidity.
Here is also a shorter pKa table for the most common functional groups in organic chemistry:



I would like to understand something: Ketones hydrogens have a pka of 19, but in aldol reactions, NaOH is usually used to deprotonate it. How is that possible if the pka of the conjugate base is lower?
Yes, the pKa values indicate that the deprotonation of the carbonyl compound is unfavorable. However, keep in mind that when we are saying a given base/acid is not strong enough to (de)protonate another compound, it is not a black and white situation. These are reversible reactions and how far the equilibrium lies to the right depends on the pKa values. In this case, they are close enough to establish an equilibrium where the little amount of enolate ion is still sufficient to react with the aldehyde/ketone that is not deprotonated. Once reacted, a new portion of the enolate is formed which also performs a nucleophilic attack on the carbonyl group. This does not, however, continue till the ketone is completely gone because the reverse reaction also takes place and the whole process is an equilibrium system.
It is the presence of both aldehyde/ketone and the enolate at the equilibrium that allows for the aldol reaction to occur. If the base was very strong, for example, LDA, the ketone would have entirely been converted into the enolate, and no further reaction would occur. To perform an aldol reaction on this enolate, a new batch of the aldehyde (or another aldehyde is added which is called crossed aldol reaction). This is only possible when a very strong base is used otherwise an equilibrium containing the two aldehydes and their enolates is established which yields a synthetically not useful mixture.
This probably belongs more in the discussion section of the aldol reaction, so I will leave the links below for further reading:
• Aldol Reaction – Principles and Mechanism
• Crossed Aldol And Directed Aldol Reactions