The Problems in Converting Esters to Ketones
Esters are carbonyl compounds susceptible to nucleophilic additions, and one may think of converting them to ketones using Grignard, organolithium, or organocuprate reagents. However, the problem with those is that Grignard and organolithium perform two consecutive additions to the carbonyl group, thus converting esters to alcohols, while organocuprates are unreactive towards esters:
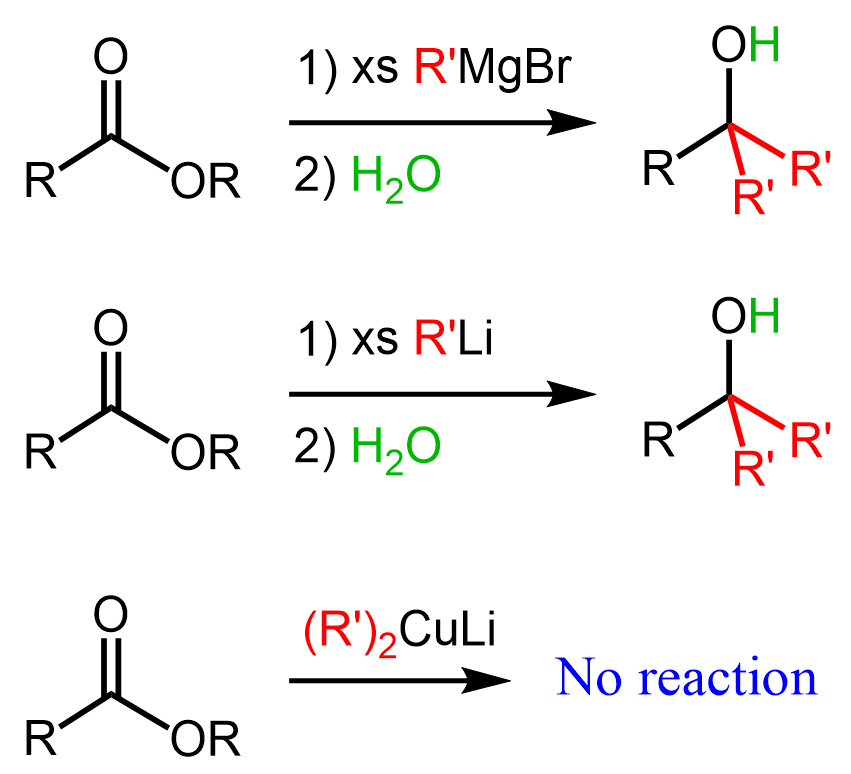
The reason for two consecutive additions of organ magnesium and organolithium reagents is the formation of a ketone after the first addition. Remember, ketones are more reactive than esters, and therefore, as soon as the ketone is formed, it reacts with the organometallic even if one equivalent of the latter is used:
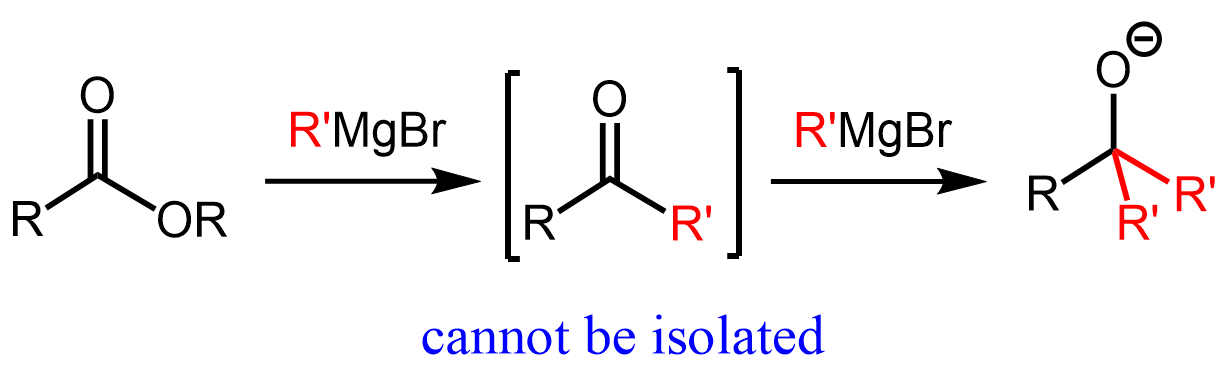
You can find the mechanism and more details about the reactions of esters and acid chlorides with organometallics in this article.
The less reactivity of the ester is explained by the delocalized lone pairs of the oxygen which are in resonance with the carbonyl and thus reducing its partial positive charge:

This effect is more profound in amides which makes them the least reactive among carboxylic acid derivatives:

Converting Esters to Ketones
Alright, we have talked about the problems of converting esters to ketones. So, what is the solution then?
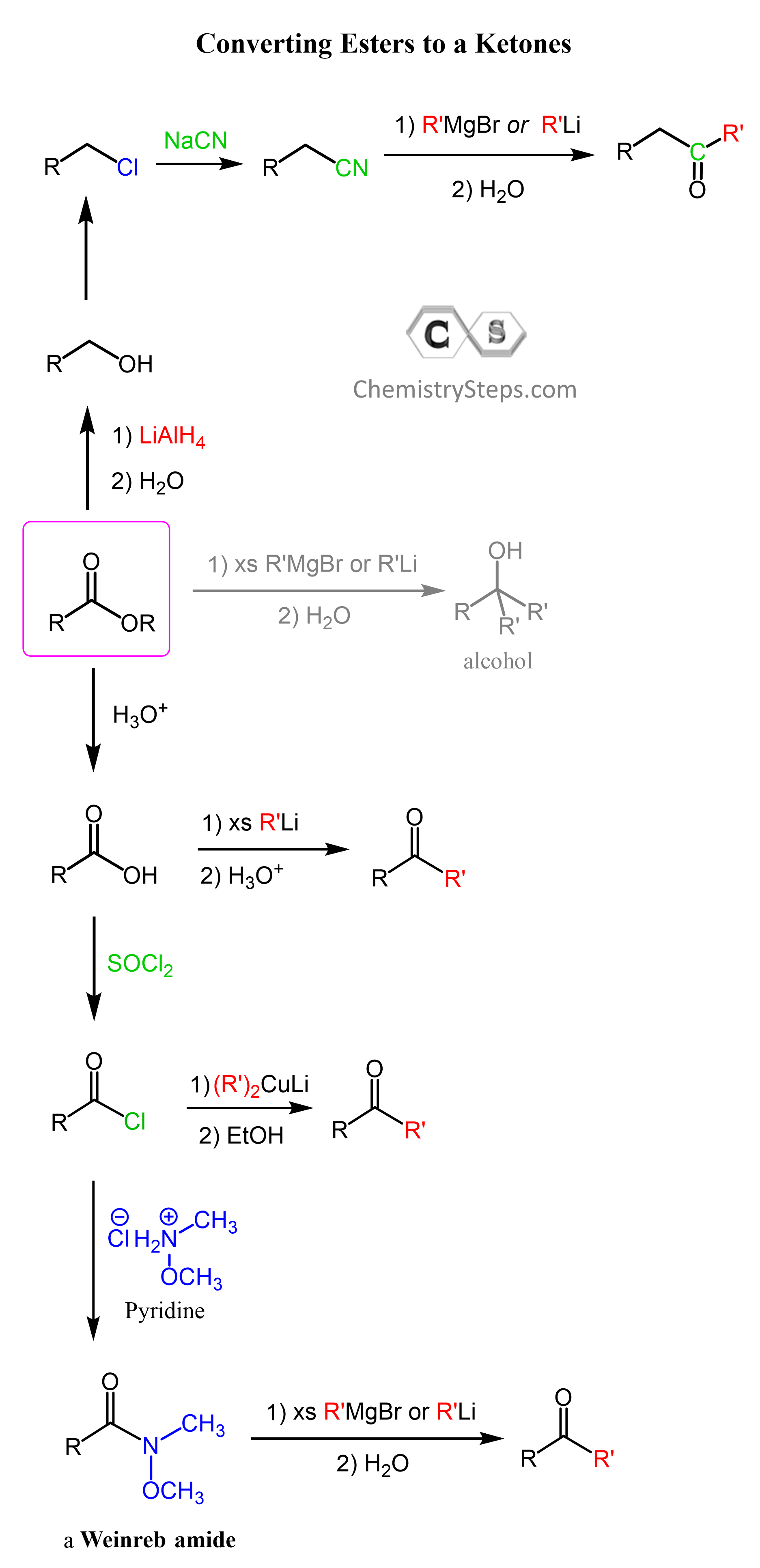
The main strategy for preparing ketones from esters is the conversion of esters to the corresponding carboxylic acids via acid or base catalysis:

Unlike esters, the reaction of carboxylic acids with an excess of organolithiums does not produce an alcohol. Instead, after the deprotonation, a carboxylate ion is formed, and the powerful nucleophilicity of the organolithium allows for another nucleophilic addition to the carboxylate ion. This addition forms a tetrahedral dianion intermediate which lacks a relatively good leaving group and stays in the solution until water is added to hydrolyze it to the corresponding ketone:

Notice that Grignard reagents are not as nucleophilic and do not react with the carboxylate ion, so we can not use them to convert carboxylic acids to ketones. More about the conversion of carboxylic acids to ketones can be found here.
Esters to Ketones via Acid Chlorides
If you do not have the needed organolithium, an organocuprate can be used by first converting the acid to acid chloride. Acyl chlorides can be prepared by reacting carboxylic acids with thionyl chloride (SOCl2), phosphorous trichloride (PCl3), or phosphorous pentachloride (PCl5):

Unlike, esters, ketones, and especially carboxylic acids, acid chlorides are very reactive and can be converted to ketones by reacting with organocuprates:

Once again, the difference between Grignard and organolithiums is that organocuprates do not react with the ketone that is formed after the first nucleophilic addition. The mechanism of the reaction between acid chlorides and organocuprates is discussed here.
Esters to Ketones via Weinreb Amide
Now, if you have an acid chloride but an organocuprate, you can still use, for example, a Grignard reagent, only this time, the acid chloride must be first converted into a Weinreb amide (N-methoxy-N-methyl amides):

The first step is the nucleophilic addition of the organolithium or organomagnesium to the amide. The tetrahedral intermediate, formed after the addition of organolithium or organ magnesium (Grignard) reagents to the Weinreb amide, is stabilized by chelation of the magnesium atom by the two oxygen atoms.
Esters to Ketones via a Nitrile
Another option for converting an ester to a ketone is this longer path which would also add a carbon atom:
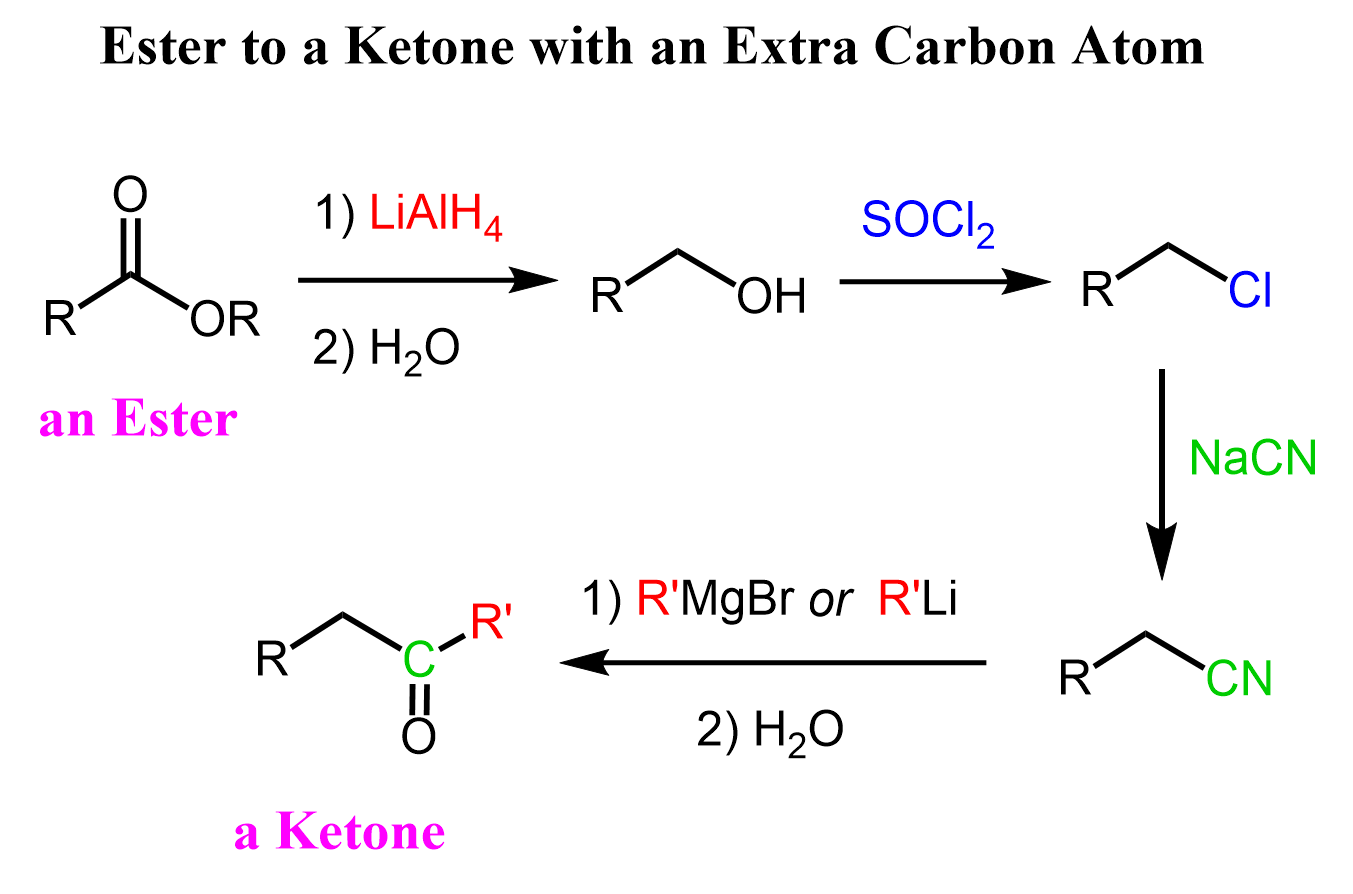
First, we reduce the ester to a primary alcohol using LiAlH4, then convert the alcohol to a halide with either an HX acid or thionyl chloride (SOCl2), convert the alkyl halide to a nitrile via an SN2 substitution with a cyanide ion (–CN), and finally react the nitrile with an organolithium or an organomagnesium. For the mechanism of the reaction of nitriles with organometallics, you can read this article.
In the end, let’s put a summary of the problems and ways of converting esters to ketones:
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz

