In this chapter on alpha carbon chemistry, you are going to come across the term “enol” almost in any new reaction. So, what is an enol? As the name suggests, these are species that contain an alkene (ene) and an alcohol (ol). More specifically, these functional groups are connected, meaning the OH group is connected to the C=C double bond:

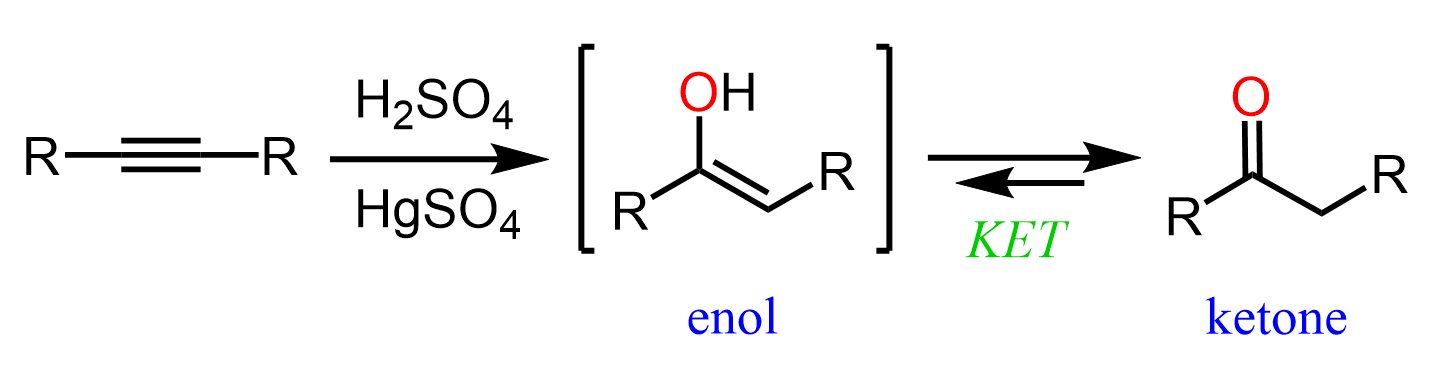
What makes this combination interesting is that it turns out that the OH and C=C do not form a particularly stable combination, which, in other words, means that enols are quite reactive. For example, recall from the acid-catalyzed hydration of alkynes that the final product, ketone is formed through an unstable intermediate which is the enol form of the ketone:
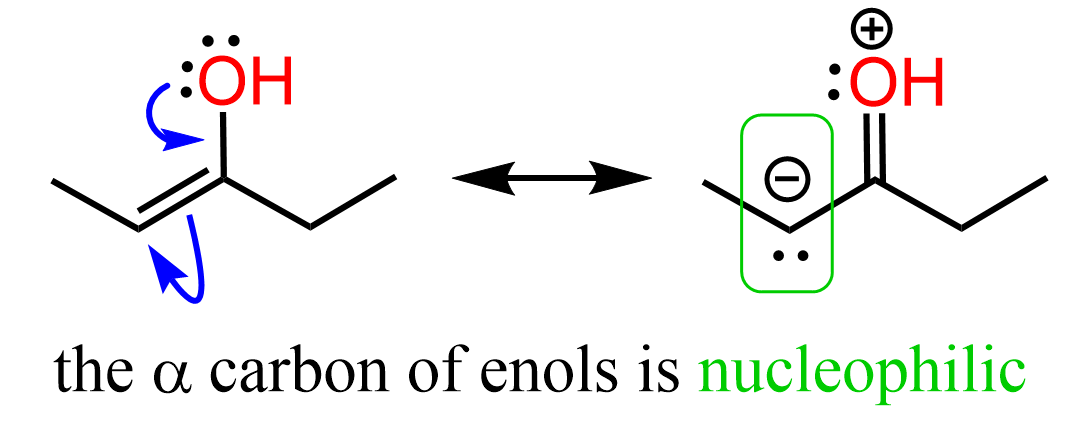
The reactivity of enols comes from their electron-rich nature. We have a double bond connected to an atom capable of resonance-donation, and if it encounters an electrophile, a type of a nucleophile-electrophile reaction will occur.
For example, enols can be halogenated, and if we mix an aldehyde or a ketone with a halogen in the presence of an acid or base, an alpha halogenation occurs:
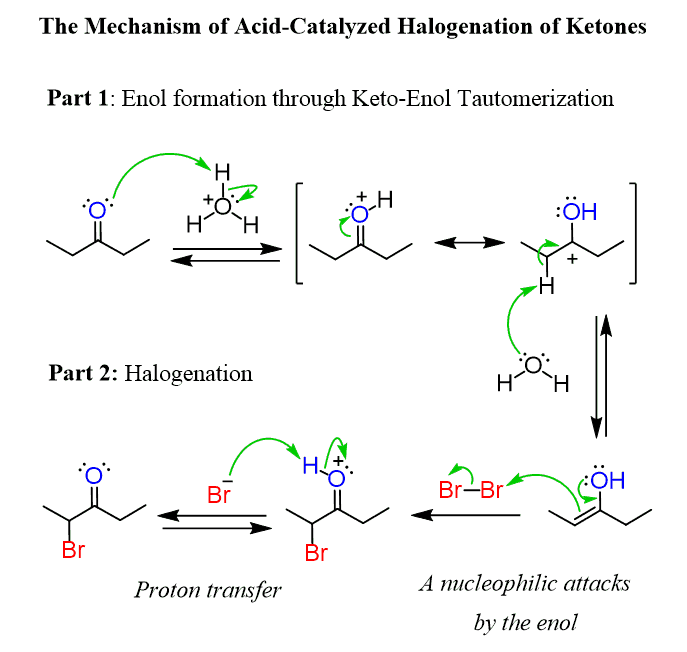
Notice that we mentioned that the reaction takes place in the presence of either acid or a base, and that is because both acidic and basic conditions catalyze the interconversion of aldehydes and ketones to their enol forms. This interconversion is what is called keto-enol tautomerization (KET) which is an equilibrium process where the ketone is present in a much greater quantity. The enol is a constitutional isomer of the corresponding aldehyde or the ketone, and they are said to be tautomers, thus the name keto-enol tautomerization.

In a broader definition, keto-enol tautomerization pertains not only to the equilibrium between a ketone and its tautomer enol but also to any carbonyl compound such as aldehydes, esters, amides and etc. and its enol form. For example, the enol form of amides is the amidic acid which, as we have seen in the hydrolysis of nitriles, tautomerizes to the amide. The tautomerization mechanism can be shown in two proton-transfer steps starting with a protonation of the nitrogen:

It is worth mentioning that even meticulously cleaned glassware contains a trace amount of an acid or a base which is enough to facilitate keto-enol tautomerization, thus there is always a certain percentage of the enol present in the solution.
The Mechanism of Keto-Enol Tautomerization
You are probably reading this article as part of the alpha carbon chemistry, which means you are already well familiar with resonance structures and wouldn’t confuse them with tautomers. Remember, resonance structures are different Lewis structures of the same compound, and they are separated with double-headed arrows. Tautomers are different compounds: they are constitutional isomers and can sometimes be separated or locked in one form to be characterized and studied with different techniques. The keto-enol tautomerization is a chemical reaction, catalyzed by an acid or a base catalyst. So, let’s see it happens starting with the acid-catalyzed tautomerization.
Acid-Catalyzed Keto-Enol Tautomerization
The first step is the protonation of the oxygen by the acid forming a resonance-stabilized oxocarbenium ion. This is essentially the activation of the carbonyl which now makes it possible to deprotonate the alpha position. This can be done by a water molecule:

The rate-determining step of acid-base keto-enol tautomerization is the deprotonation of the protonated ketone.
The reverse reaction starts with the protonation of the double bond as it is electron-rich because of the electron-donating resonance effect of the oxygen. Recall from the reactions of alkenes that regular alkenes without an electron donation group are also capable of undergoing protonation, but enols are much more nucleophilic.

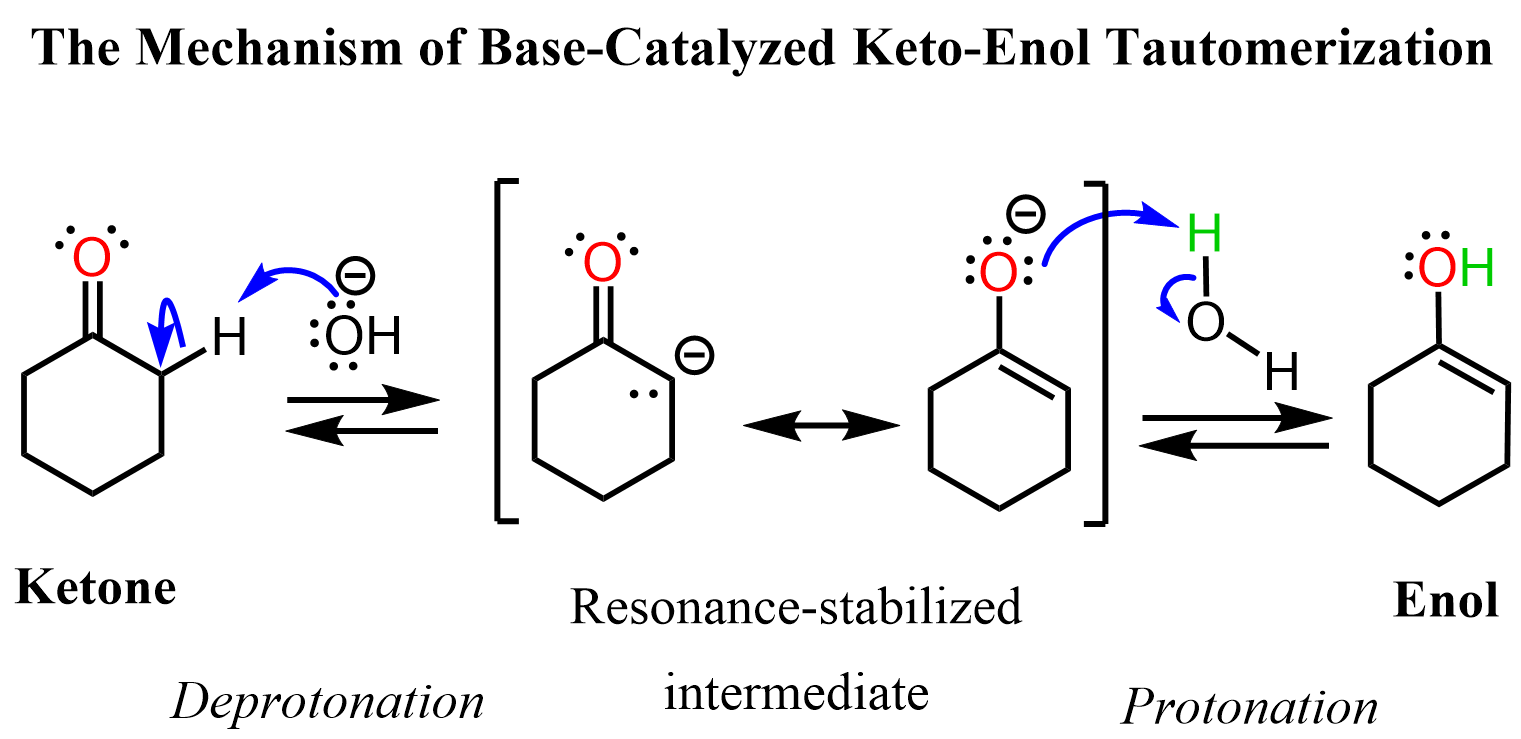
Base-Catalyzed Keto-Enol Tautomerization
The base-catalyzed keto-enol tautomerization starts with the deprotonation of the alpha carbon forming the corresponding enolate which is then protonated by water to the enol form:
One question you may wonder here is how a weak base such as sodium hydroxide can deprotonate the ketone which has a pKa value of ~20. And just like any reaction, there is always an equilibrium where both reactions occur, and what matters is the value of the equilibrium constant. For example, the equilibrium constant for the keto-enol tautomerization of acetone is only 5 x 10-9, and that of acetaldehyde is 6 x 10-7:
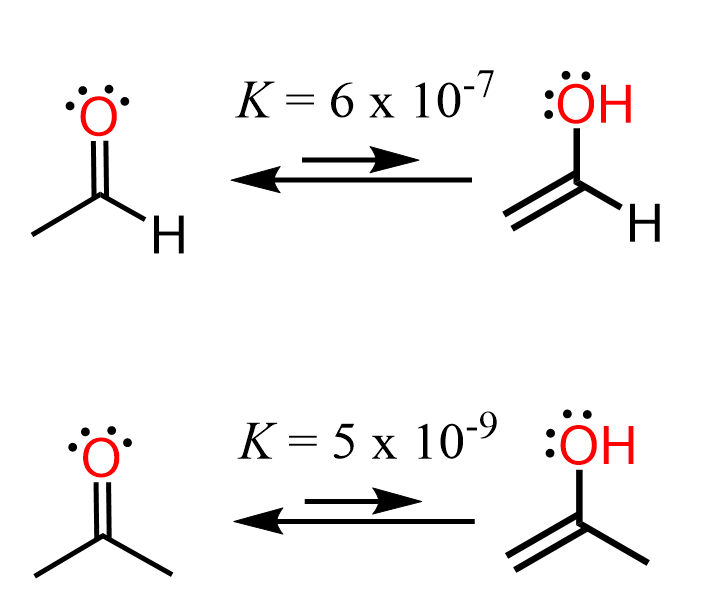
The reason for the greater tendency of aldehydes to form enols is that ketones themselves are more stable than aldehydes as the electron-donating alkyl groups stabilize the partial positive charge of the carbonyl carbon.
The Regiochemistry of Keto-Enol Tautomerization
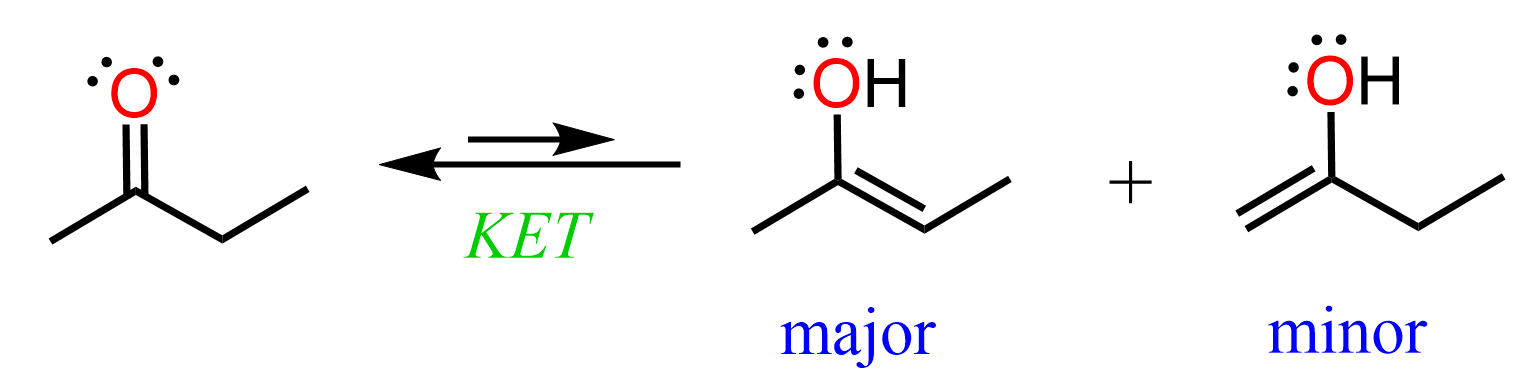
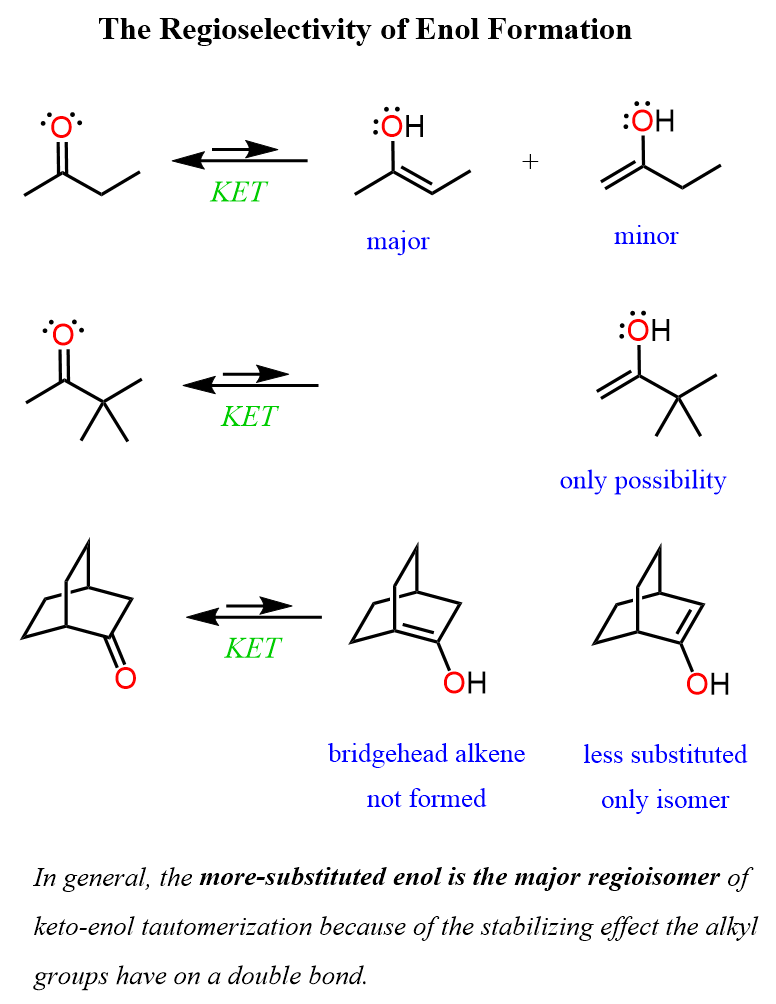
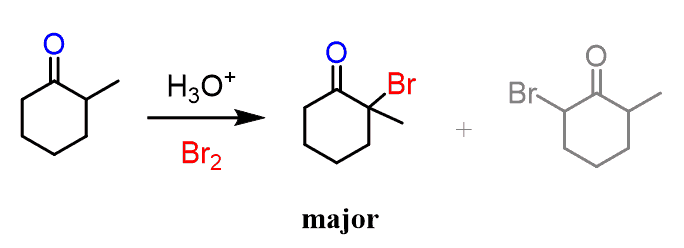
In general, the more substituted enol is the major regioisomer of keto-enol tautomerization because of the stabilizing effect the alkyl groups have on a double bond. We have already seen this feature in the stability of alkenes.
The less substituted enol is formed when the other isomer cannot because of lacking α protons or geometric infeasibly:
The less substituted enol can also be prepared if a sterically hindered base such as LDA is used. This is a strategy that is used in enolate synthesis rather and you can read more about this here:
One of the techniques to study keto-enol tautomerization is the use of D2O as a solvent as it replaces the protons of the alpha carbon with deuterium:

It has been shown that the exchange rate of the CH2 and CH3 protons in 2-butanone is 4.2:1, considering their ratio which plays in favor of the CH3 protons (Sundberg, Carey – Advance Organic Chemistry, part A).
The regiochemical preference of the more substituted enol can also be seen for example in the halogenation of ketones:
Keto-Enol Equilibrium and Stability of Enols
We have mentioned that the keto form is far predominant in the equilibria of keto-enol tautomerization.
The preference of the enol form is mainly seen for molecules where intramolecular hydrogen bonding or conjugation and aromaticity is possible. The most common case of intramolecular hydrogen bonding is observed in β-diketones. For example, acetylacetone exists 85% in the enol form because of the intramolecular hydrogen bonding:

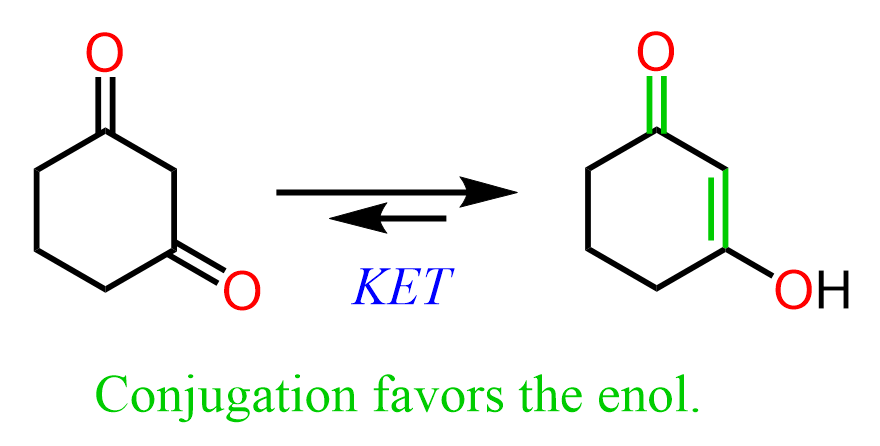
Conjugation is another factor that may favor the enol form. For example, in the following enol form, the OH is too far from the carbonyl to form hydrogen bonding, but the double bond is conjugated with the carbonyl making 1,3-cyclohexanedione the minor species in the mixture:
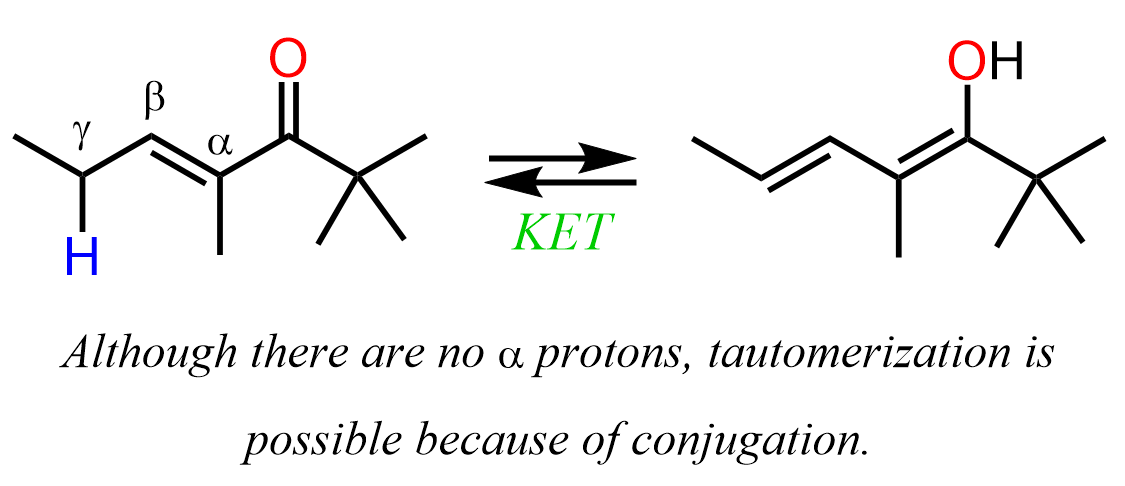
The effect of conjugation can also be seen when a carbonyl compound with no α hydrogens tautomerizes by losing a distant proton:
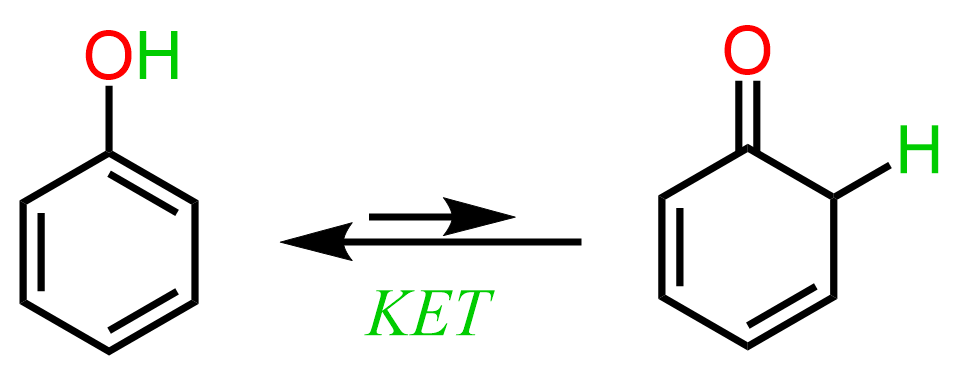
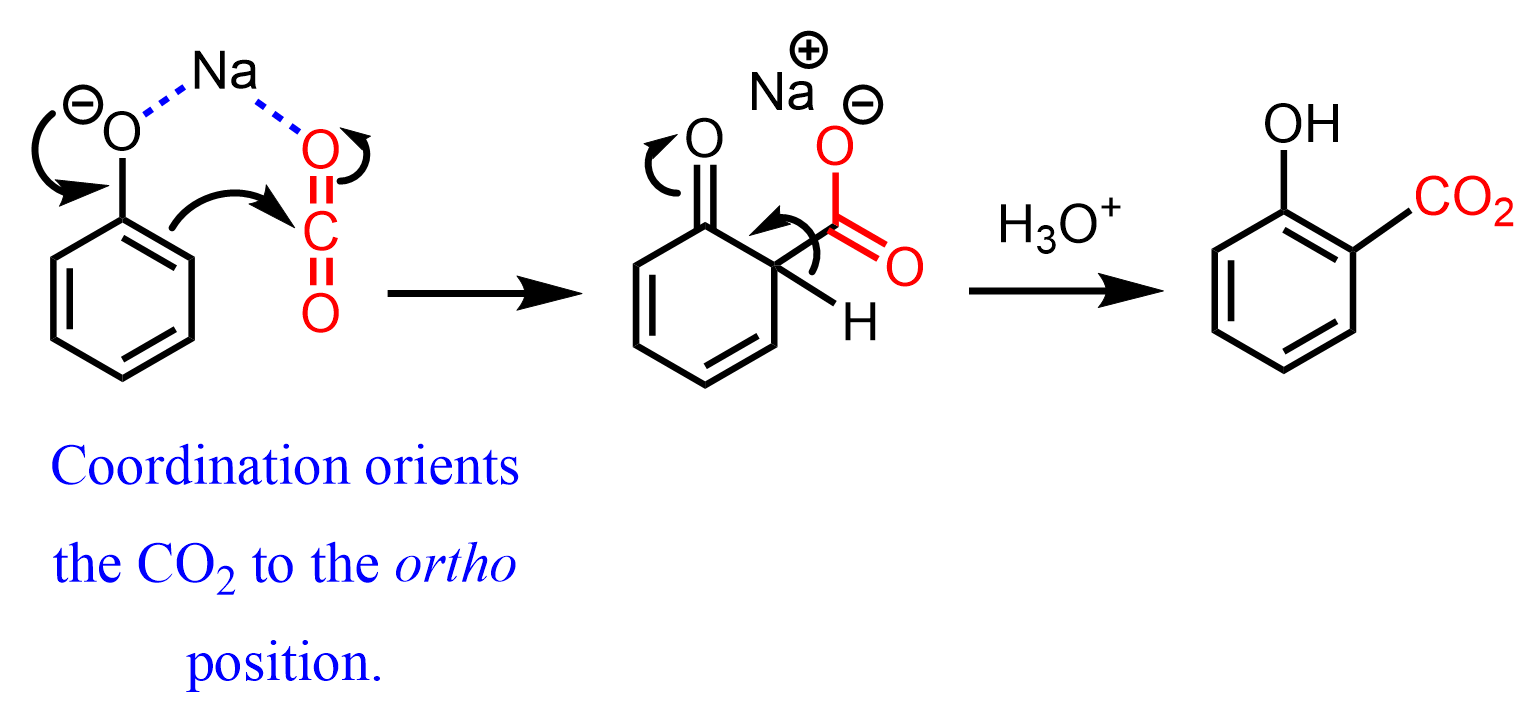
Continuing this topic, let’s finally discuss how aromaticity affects the keto-enol equilibria. Aromaticity is a type of conjugated system associated with additional stability. The best example demonstrating the effect of conjugation on the preference of enol form is phenol. It is an aromatic alcohol and we can draw its tautomer in the keto form which, however, is extremely unfavorable because it breaks the aromaticity of the molecule:
The keto form of the phenol is formed, for example, in the synthesis of aspirin when the phenol reacts with carbon dioxide via electrophilic aromatic substitution. It is quickly converted to the enol form to restore the aromaticity of the ring, and possibly hydrogen bonding of the OH with the CO2 group:
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- The Cannizzaro reaction
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Decarboxylation
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Mannich Reaction
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
