In the previous post, we learned that the anti-dihydroxylation of alkenes is achieved by converting them to epoxides, followed by acid or base-catalyzed ring-opening of the epoxide:

To convert alkenes into cis-diols by syn dihydroxylation, they are reacted with a basic solution of potassium permanganate (KMnO4) or Osmium tetroxide (OsO4):
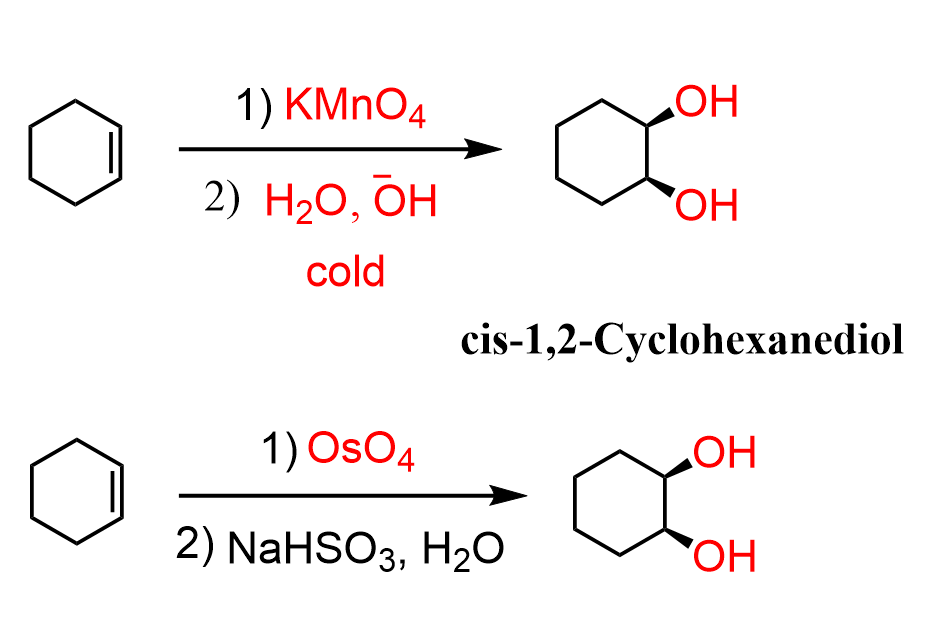
Both reactions go through the formation of a cyclic intermediate, which is formed by a syn addition to the double bond. The intermediates are then hydrolyzed by water, during which the stereochemistry of newly-formed C-O bonds is retained, thus producing cis-diols:

And here is a more detailed plausible mechanism for the syn dihydroxylation of alkenes with KMnO4.

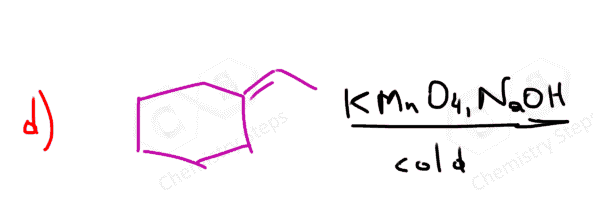
There is one thing to be careful about when using KMnO4. Since it is a strong oxidizing agent, it may cleave the C-C bond of the diol and oxidize it further to a carbonyl. In acid and neutral solutions, it always does so; hence, a basic solution of the permanganate must be used at low temperatures.
OsO4, on the other hand, is more selective for preparing cis-diols. However, it is also toxic and more expensive. As a comparison, 1 g (OsMO4) – $266, 25 g (KMnO4) – $43 in Aldrich, and you can also purchase KMnO4 from a store since it is also used as an antiseptic:
To overcome these limitations, NMO (N-methylmorpholine N-oxide) is added to the reaction, and it deoxidizes the Os6+ species back into OsO4, which can perform another oxidation of the alkene:

In the above example, a meso compound was formed, and that is because the starting alkene, cyclohexene, is a symmetrical molecule.
Keep in mind that if an unsymmetrical alkene is used, the syn dihydroxylation produces a pair of enantiomers:

Of course, if the alkene had a stereogenic center(s) that do not participate in the reaction, then a pair of diastereomers would have been formed.

Hi! I found this post very helpful. I just wanted to bring to your attention that in the second diagram depicting the syn dihydroxylation of alkenes, the image shows cis-1,2-cyclohexanediol but is described as cis-1,2-cyclopentanediol. The information is still extremely helpful, thank you!
Thank you for the kind words, Anastasia! I have fixed the notation.