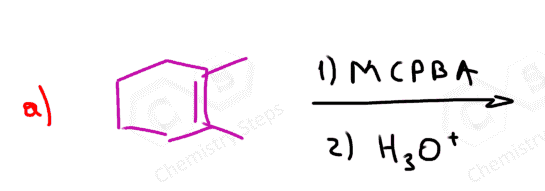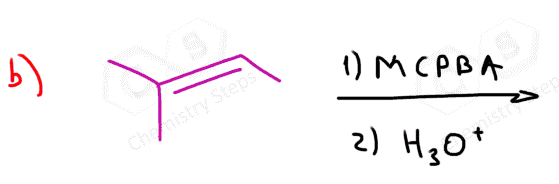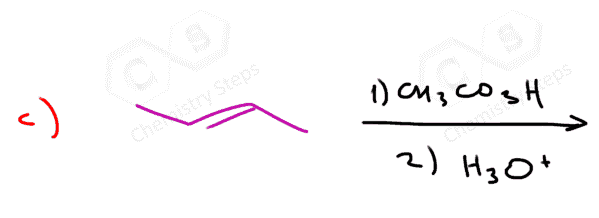Diols and Epoxidation
Dihydroxylation is the addition of two OH groups to an Alkene:

This can be achieved in several ways depending on the structure of the target diol, mainly, whether we need it in a cis or trans configuration:

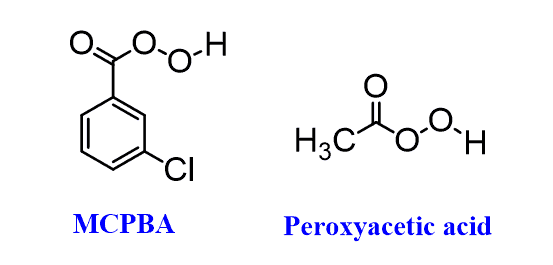
In this post, we will focus on the anti-dihydroxylation which is achieved by converting the alkene into an epoxide first. This is usually done with peroxyacids such as MCPBA (meta-chloroperoxy benzoic acid) or Peroxyacetic acid:
The mechanism of epoxidation is similar to the formation of halonium ion and oxymercuration that we discussed in the other addition reactions of alkenes.
The oxygen in the peroxide is electron-deficient and is attacked by the p electrons of the π bond. In addition, the O-O bond is relatively weak which also makes the bond-cleavage easier:

The epoxidation is a syn addition, and the oxygen can add to either side of the alkene. Therefore, a mixture of enantiomers is obtained when substituted alkenes react with acid peroxides:

As usual, watch for meso compounds as not every compound with stereogenic centers is going to be chiral. For example, when a symmetrical cis alkene is used, the corresponding meso epoxide is obtained:
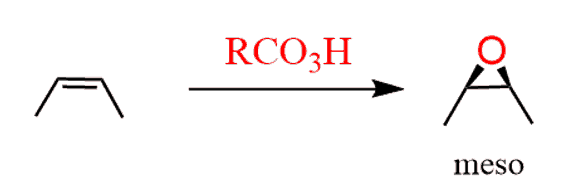
Epoxide Ring-Opening
Epoxides are highly stranded three-membered rings which are very reactive toward nucleophilic substitution reactions since they result in opening the ring thus releasing the strain:

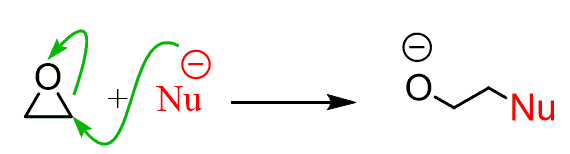
The epoxide ring can be opened under acid conditions and this is how the anti-dihydroxylation is achieved. The acid serves as catalyst by converting the oxygen into a better leaving group alcohol:
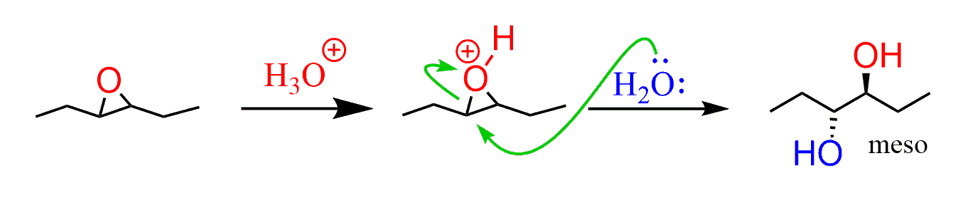
In the case of cyclohexene that we discussed earlier, a pair of enentiomeric trans-diols would be obtained:

Regiochemistry
Under acidic conditions, when a weak nucleophile is used, it attacks the epoxide at the more-substituted carbon. For example, when alcohol is used as the nucleophile, it attacks the first carbon, therefore the reaction is regioselective:
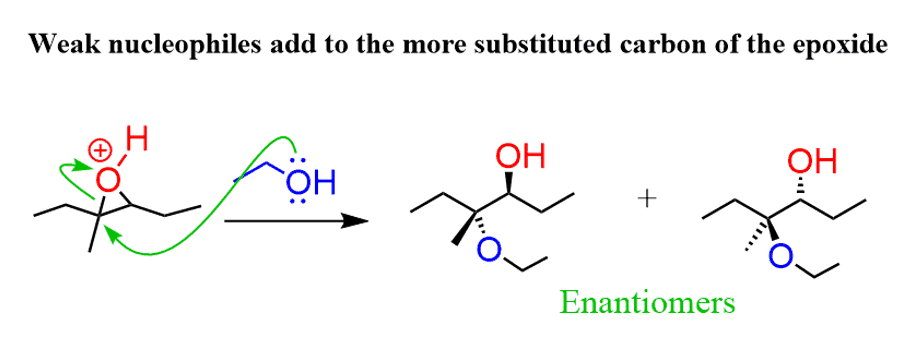
This is explained by the stability of the energy differences of the two possible transition state:
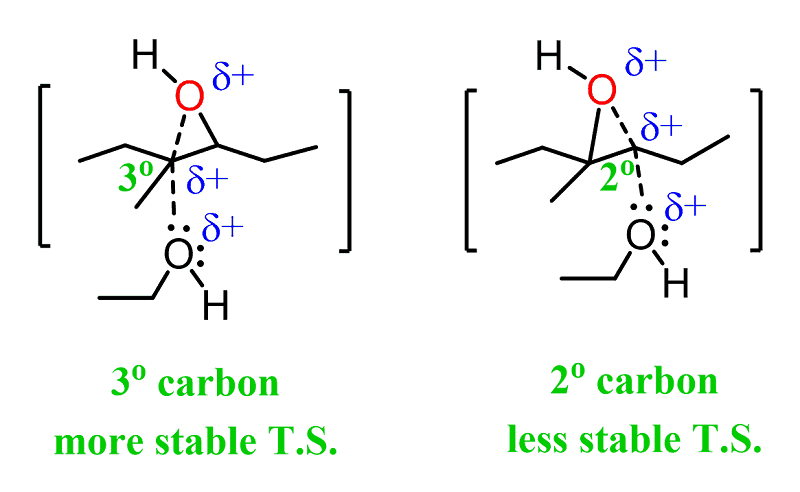
The first transition state has a partial positive charge on a more substituted carbon, making it more stable.
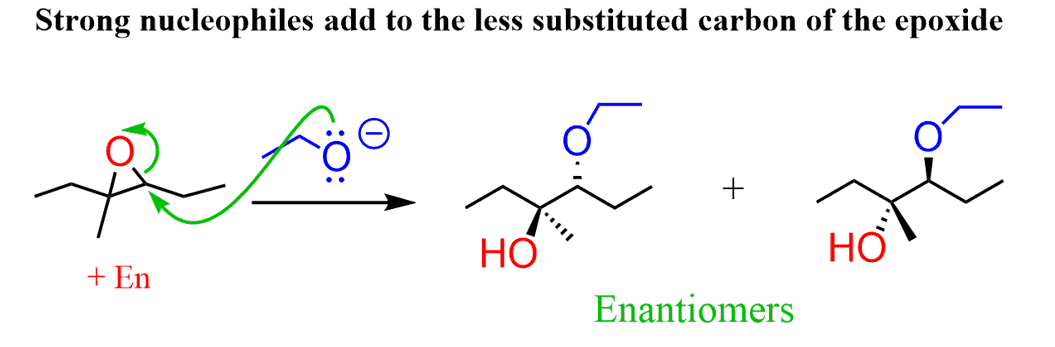
On the other hand, when a strong nucleophile is used the ring opening of an unsymmetrical epoxide occurs mainly by attacking the less substituted carbon atom:
The Stereochemistry of anti-Dihydroxylation
Most often, the anti-dihydroxylation gives a mixture of enantiomers unless the starting alkene contains a chiral center in which case, a pair of diastereomers will be formed.
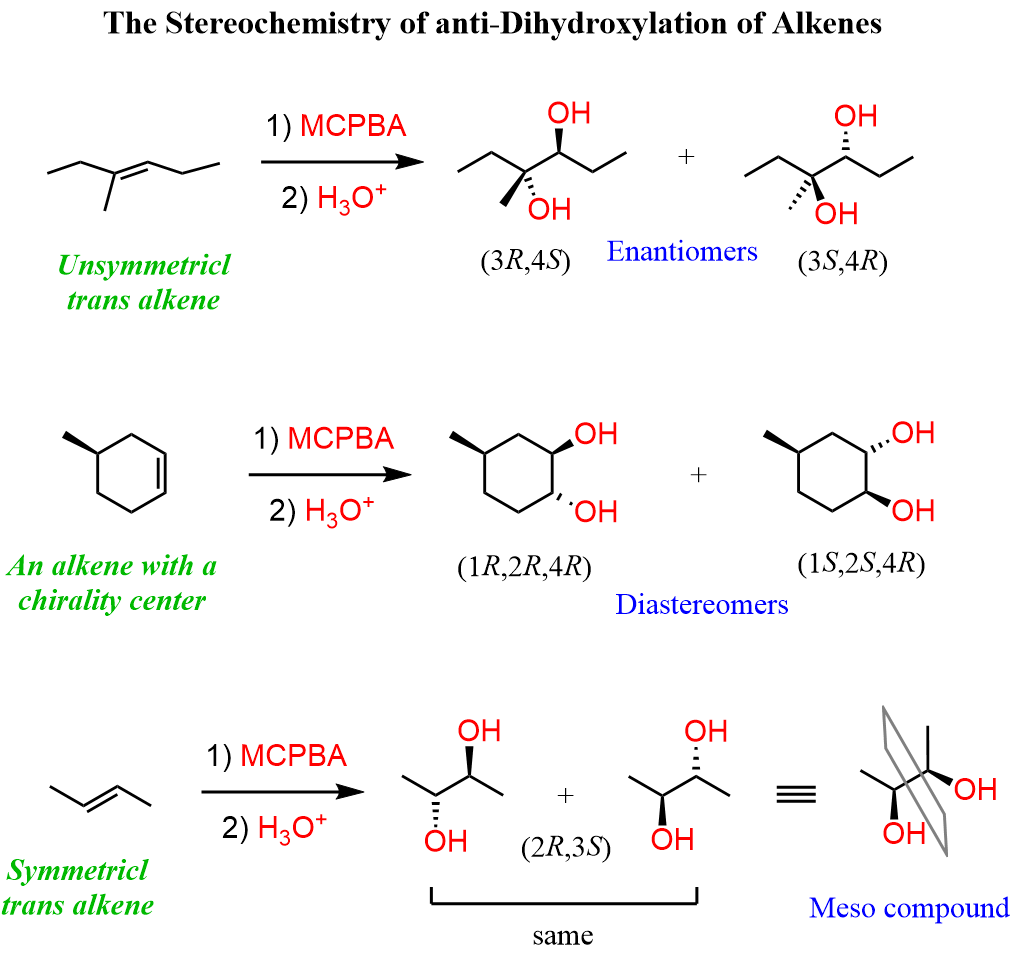
Watch out for meso compounds when working with a symmetrical alkene. Although visually may not be apparent, the dihydroxylation of a symmetrical trans alkene will give a meso compound.
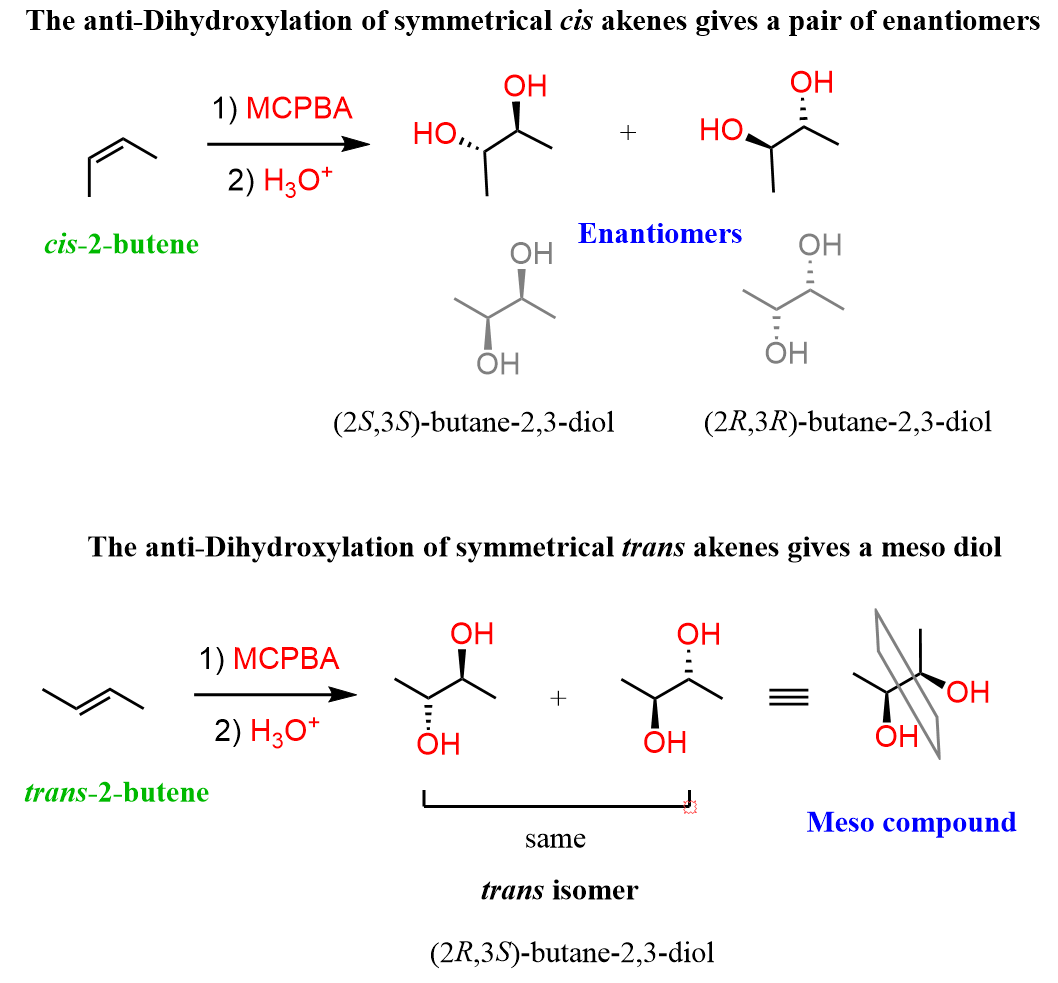
You can read the article “Cis Products in Anti Additions to Alkenes” for about this types of tricks in the addition reaction of alkenes.