In the previous post, we talked about the acid-catalyzed hydration of alkenes where an alcohol is formed as the major organic product:

If an alcohol, rather than water, is used as the solvent a very similar reaction takes place and an ether is produced.

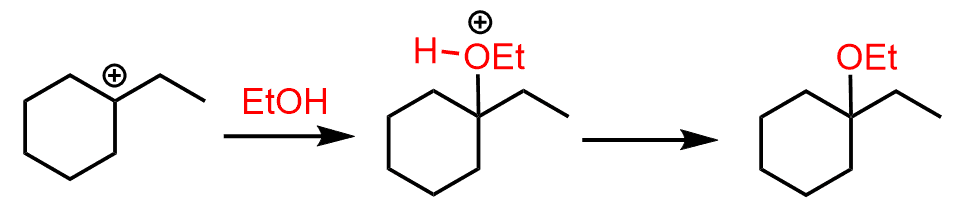
Just like the hydration, it goes through a stepwise mechanism starting with the protonation of the double bond:
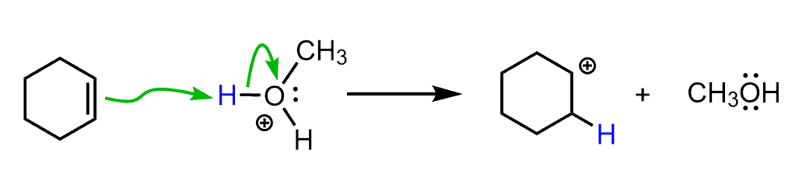
Notice that instead of the hydronium ion that we saw in the acid-catalyzed hydration, the immediate protonating species here is the oxonium ion of the alcohol. This ion is strong enough of an acid to protonate the double bond and form a carbocation. Once the carbocation is formed, it can now be attacked by the alcohol:
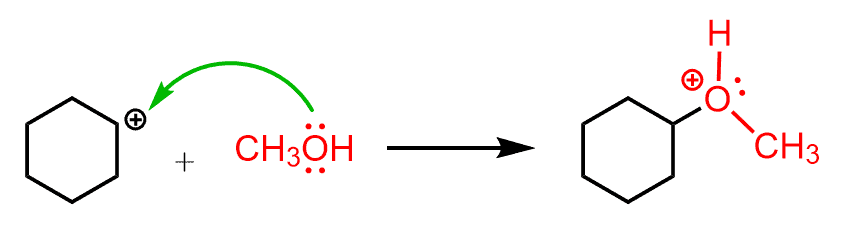
After the deprotonation of the oxonium ion, the corresponding ether is formed as the final product.
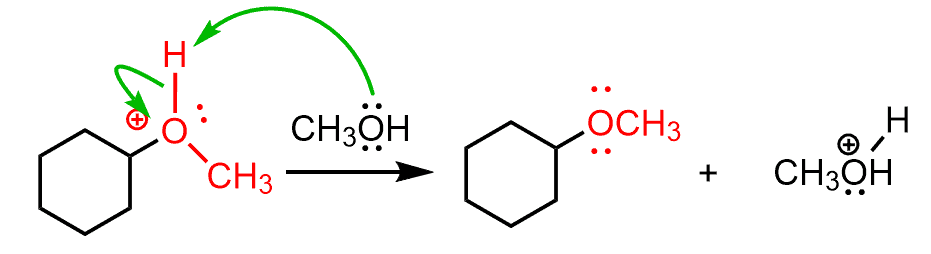
And just like in the acid-catalyzed hydration, all the steps of this transformation are reversible. We only show them with one arrow as we are interested in the forward reaction and trying to shift the equilibrium to that side.
The rate-determining step of the process sis the protonation of the double bond and therefore, the reaction can be summarized as follows:

The Regiochemistry of Alcohol Addition
The acid-catalyzed addition of methanol to cyclohexene produces one ether as the major organic product. This is because cyclohexene is a symmetrical alkene and there is no preference/difference as to where we add the OH group. So, what if an unsymmetrical alkene is used where there are two possibilities to add the methoxy group?
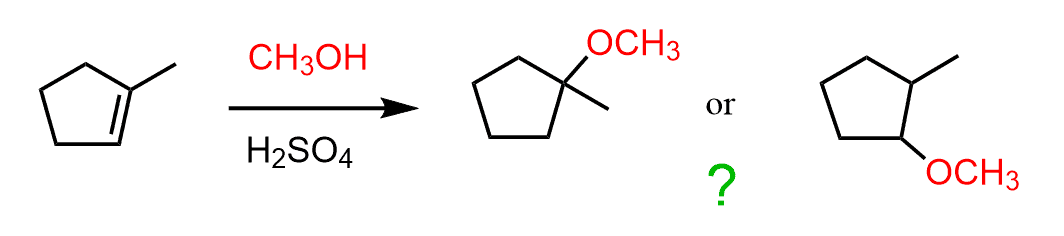
Which of the ethers is going to be the major product?
As you may have noticed, this mechanism is similar to the addition of HX acids to alkenes according to the Markovnikov’s rule. Remember, for an unsymmetrical alkene, the more stable carbocation determines the major product of the reaction:
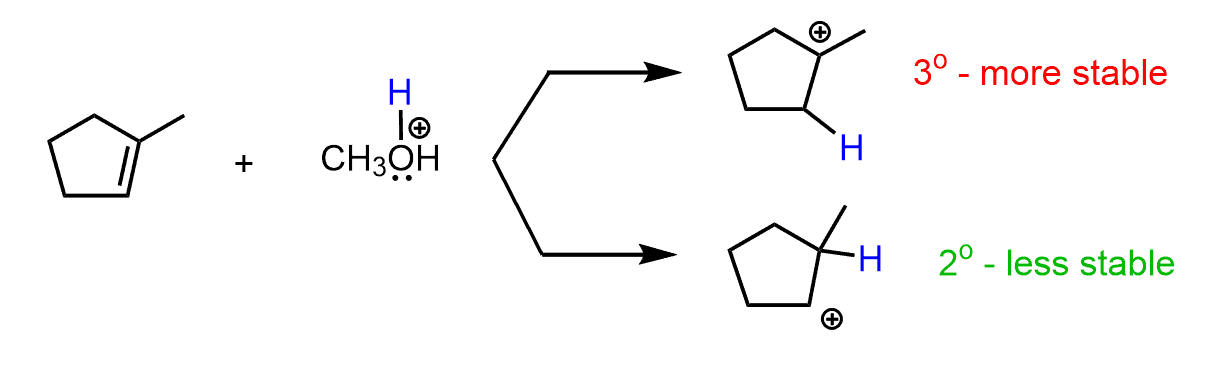
In this case, the first carbocation is the more stable intermediate and dictates formation of the corresponding ether:
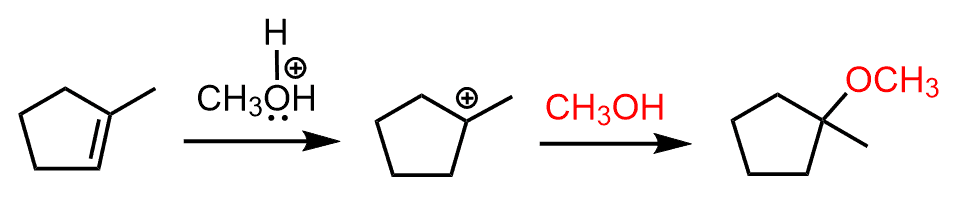
So, in summary, the regioselectivity of acid-catalyzed hydration indicates that the OR (alkoxy group) goes to the more substituted carbon.
Rearrangements in Alcohol Addition to Alkenes
Formation of carbocation intermediates in acid-catalyzed addition of alcohols to alkenes brings the possibility of rearrangements just like we have seen in SN1 and E1 reactions.
For example, the major product of the following alcohol addition reaction is not the ether with the alkoxy group on the more substituted carbon of the double bond based on the Markovnikov’s rule:
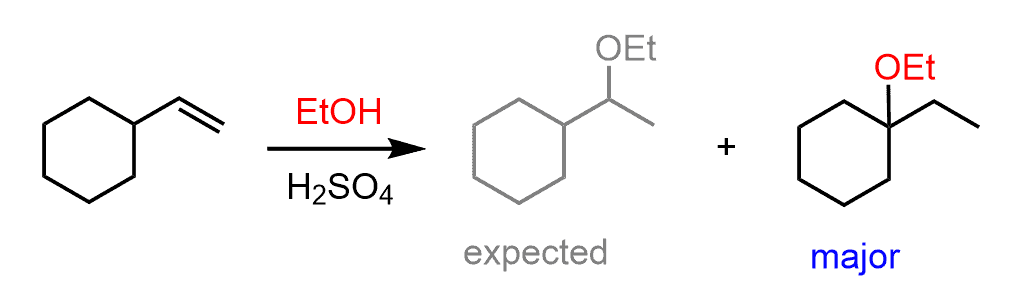
The secondary carbocation that is formed after the protonation of the double undergoes a hydride shift rearrangement to form the more stable tertiary carbocation:
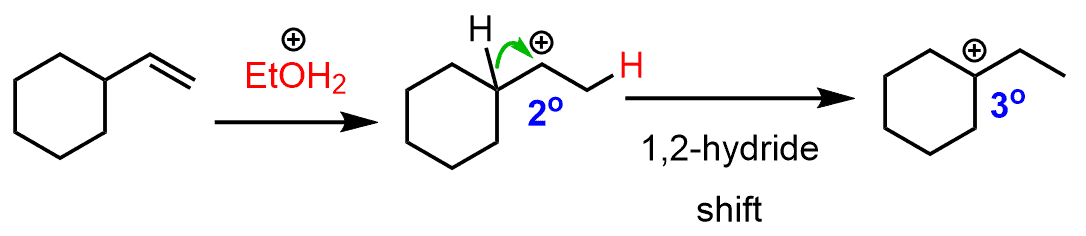
The nucleophilic attack of the ethanol results in an ether with a tertiary alkyl group:
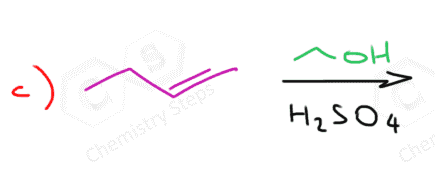
The Stereochemistry of Alcohol Addition to Alkenes
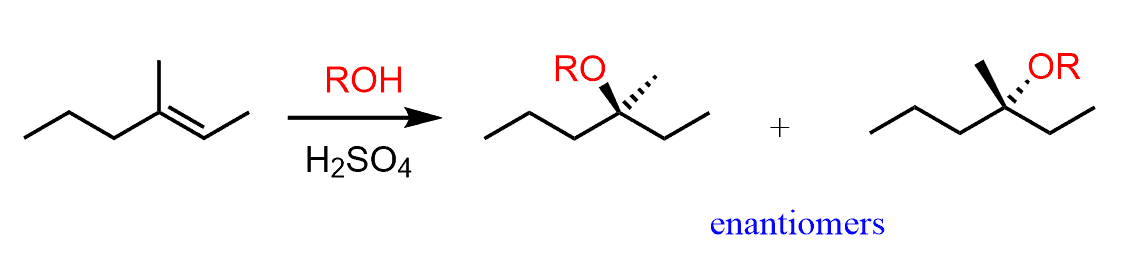
If the acid-catalyzed addition of an alcohol to an alkene produces an ether with one chirality center, then the products will be a mixture of two enantiomers:
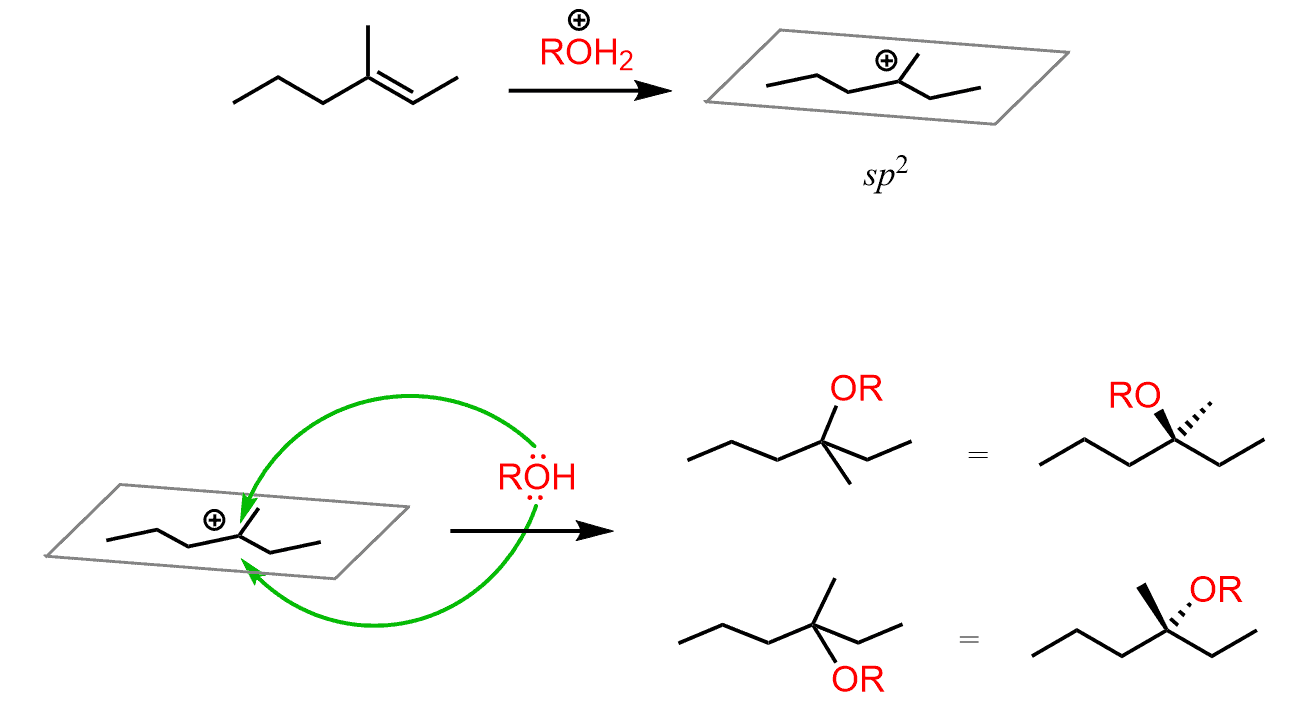
The reason for this is the same as you learned in SN1 reactions: carbocations are sp2-hybridized, flat centers (we are talking about the positively charged carbon) and the nucleophilic attack occurs from both faces:
If, on the other hand, the reaction produces more than one stereogenic center, then all the possible stereoisomers with two stereogenic centers are formed. This becomes complicated and you will most likely not need it in your class.
However, if you do, check the following post about the stereochemistry of the alkene addition reactions for the details:

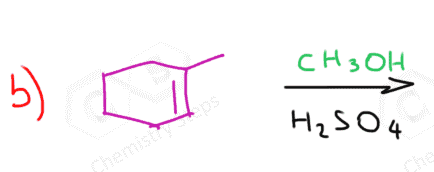
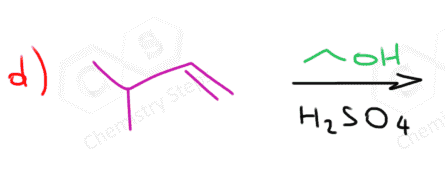
I have failed to understand the concept here… Kindly advise me I really want to move together with those of you who are doing fine in this topic.
Hello,
Please let us know if there is anything that you would like to have more explanation on.