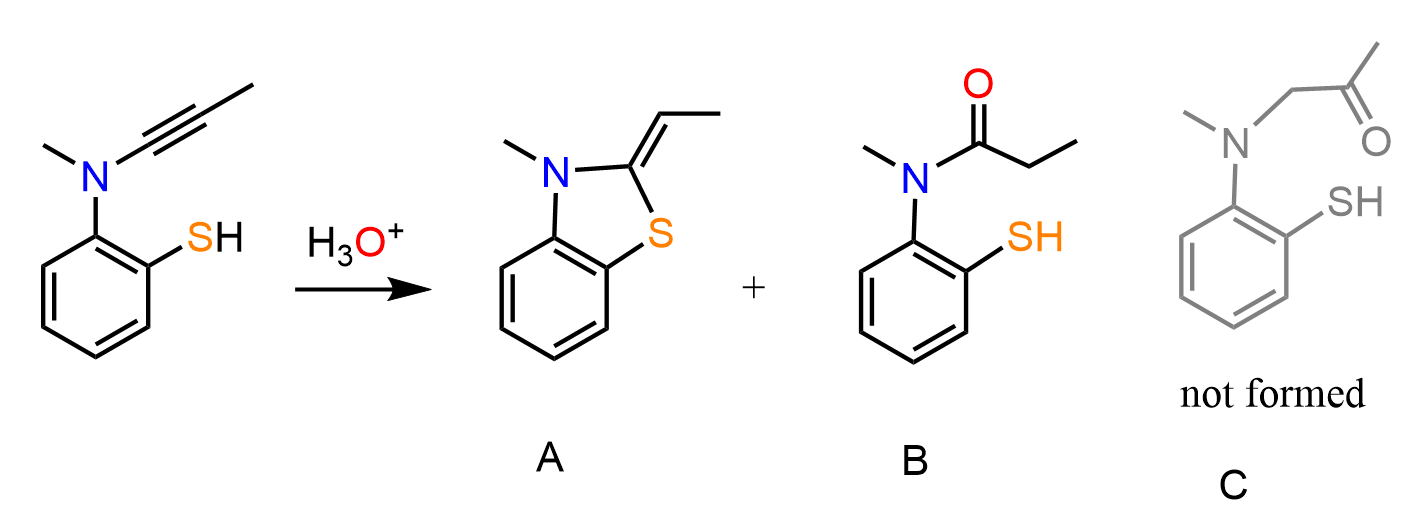
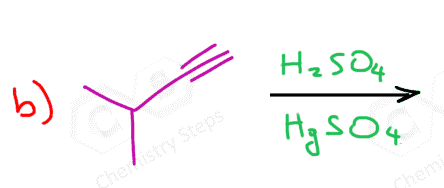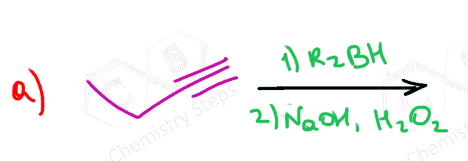In the initial discussion about electrophilic additions to alkynes, we saw that in many ways, these reactions are similar to those of alkenes. For example, in hydrohalogenation reactions, both alkenes and alkynes are first protonated forming carbocation intermediates which are then attacked by the nucleophilic halide ion:
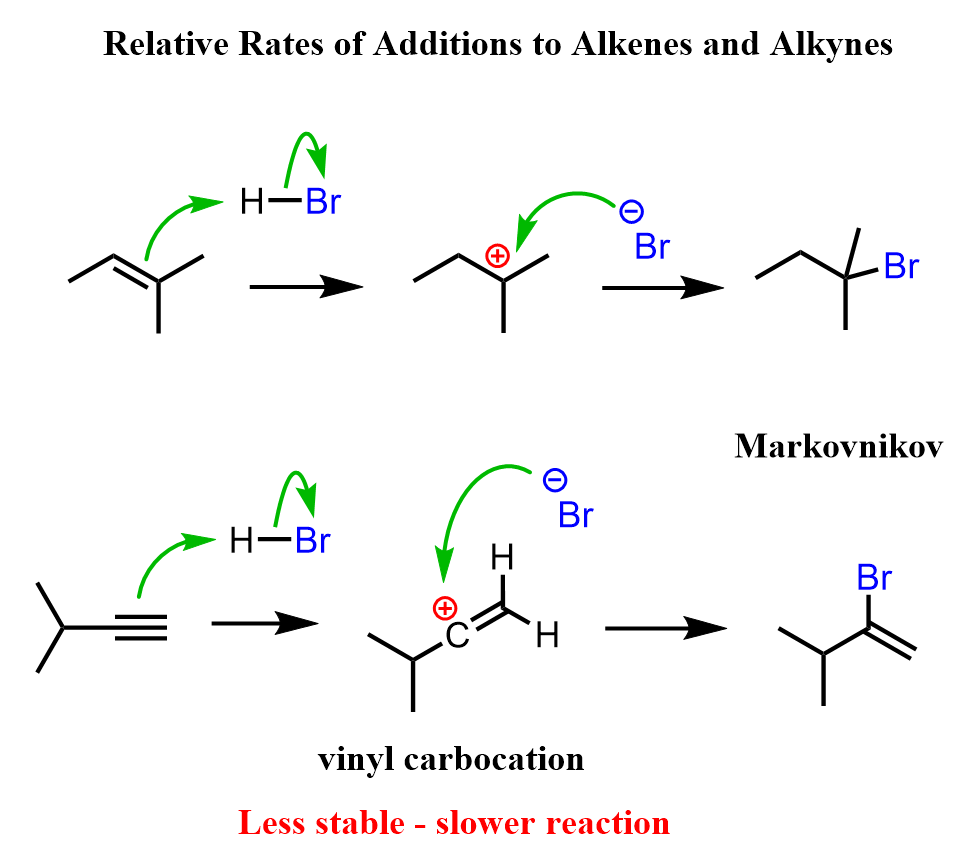
An important difference is that in the case of alkenes, a “regular” carbocation with an sp2-hybridized carbon atom is formed, whereas the protonation of the triple bond forms an sp-hybridized vinyl carbocation, which is much less stable. Because of this lower stability, the electrophilic addition reactions to alkynes are generally slower than alkenes.
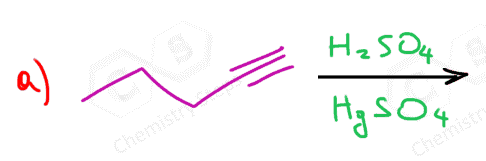
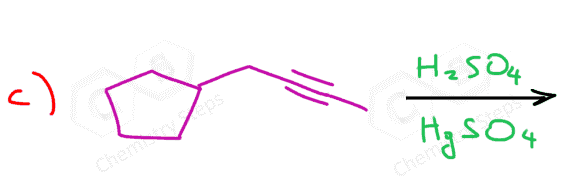
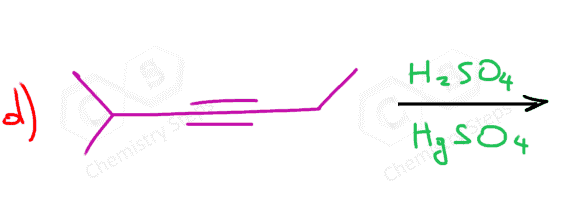
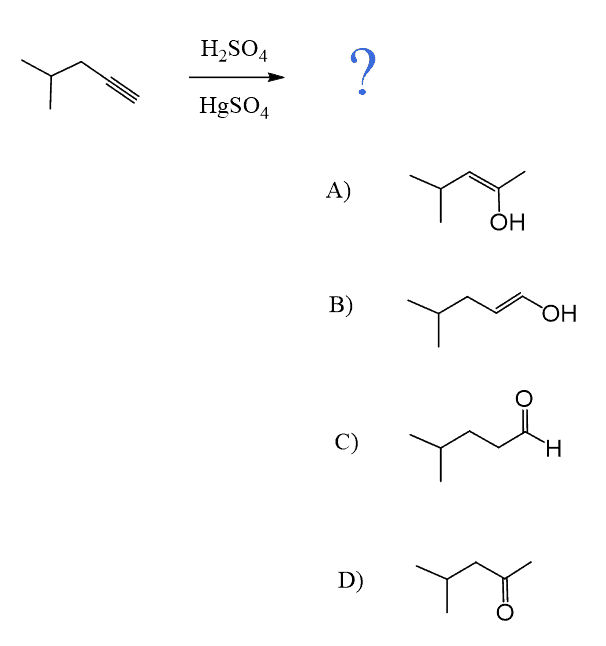
Another important reaction that starts with the protonation of the π bond is the hydration of alkenes and alkynes. There are a couple of key differences we need to address when comparing these two, and one of them is the occasional use of HgSO4 for the hydration of alkynes. The reason for using a mercury catalyst is the slower reaction of alkynes that we mentioned above. So, let’s discuss both methods for the addition of water to alkynes one by one.
Acid-Catalyzed Hydration of Alkynes
In the first, rate-determining step, the triple bond is protonated, forming the more substituted vinyl carbocation, which is attacked by water, forming an intermediate called an enol. The enol is then transformed into a ketone via keto-enol tautomerization:
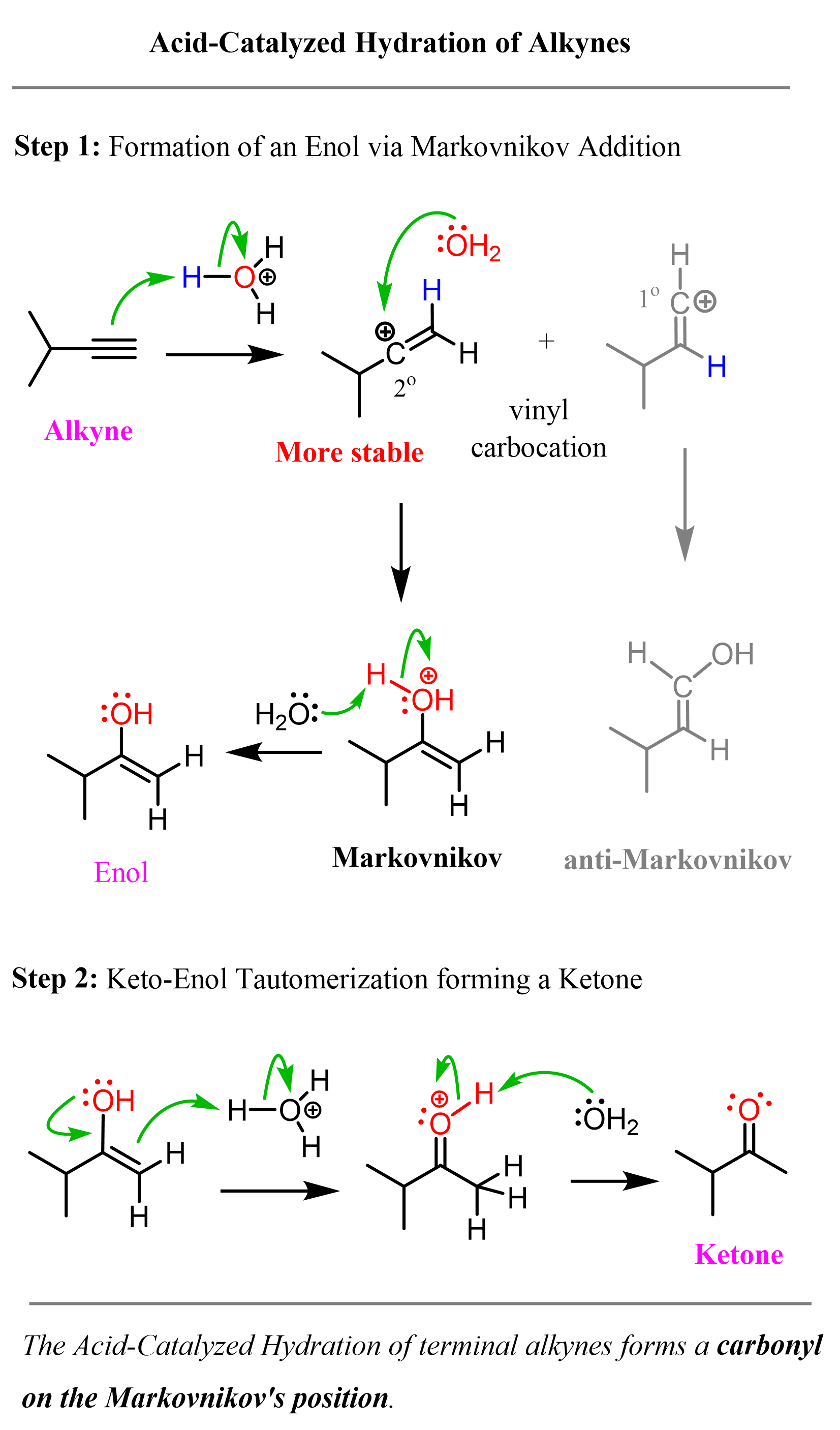
Notice that the final product of acid-catalyzed hydration of alkynes is a ketone and not an alcohol, like we saw for alkenes. The reason for this is that the enol intermediate, formed after the addition of water, is unstable and is quickly converted into a ketone. There is a separate article about enols and keto-enol tautomerization that you can read, but let’s quickly mention what enols are and why they are not particularly stable.
An enol is a molecule that combines an alkene (ene) and alcohol (ol):

We already know that alkenes are electron-rich, and it turns out that having an OH group on them increases the electron density even more via resonance donation. As a result, enols are very reactive and they interconvert to ketones or aldehydes on their own. You can view KET as a proton transfer from the OH group to the C=C double bond. This converts the C-OH into a C=O, and the C=C into a C-C bond:
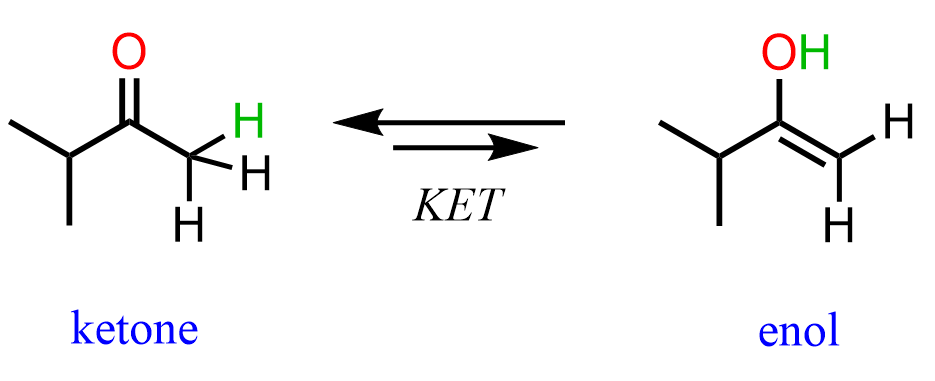
Notice that ketones and enols are different compounds that are in equilibrium, and thus we show keto-enol tautomerization with a double arrow. Do not mix this with resonance structures, which are just different representations of the same compound shown with a double-headed arrow. Aldehydes and ketones are constitutional isomers to their corresponding enols.
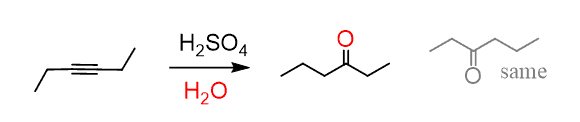
As a shortcut to acid-catalyzed hydration of terminal alkynes, remember that it forms a carbonyl in the Markovnikov’s position, thus the final product is a ketone:

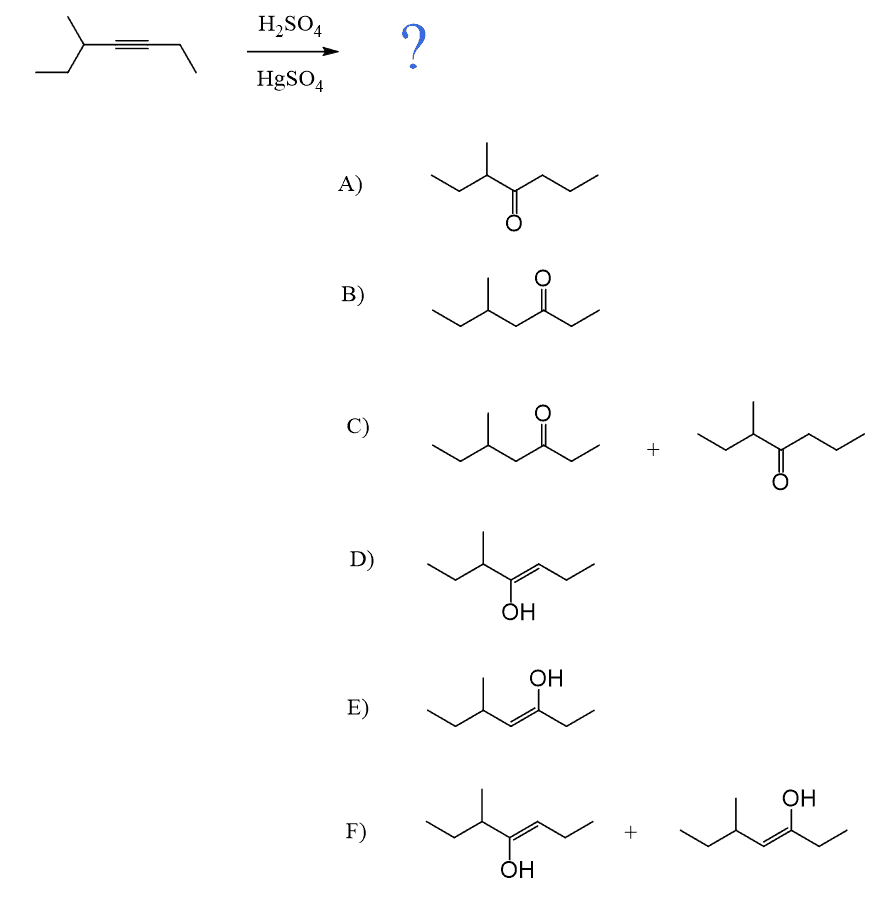
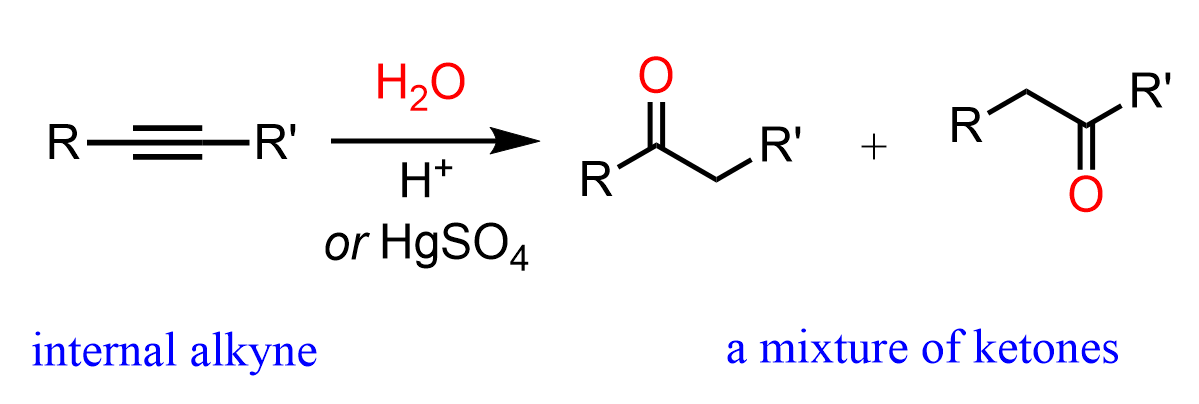
Internal alkynes give a mixture of two ketones, as there is no regioselectivity in the formation of the vinyl carbocation, as both have equal stability:
The last structural possibility of the alkyne is having two identical R groups. These are symmetrical alkynes, and they can only form one ketone:
HgSO4 for the Hydration of Alkynes
As mentioned earlier, alkynes are generally slower in electrophilic addition reactions, including hydration. Therefore, a mercury salt such as HgSO4 is sometimes used to facilitate the conversion of alkynes to ketones.
This is the oxymercuration-demercuration of alkynes, which we also saw for alkenes, however, for alkenes it was used to prevent possible rearrangements of the intermediate carbocations in acid-catalyzed hydration.
In both cases, the reaction goes via a three-membered cyclic intermediate called mercurinium io,n which is attacked by water at the more substituted carbon, thus giving a ketone instead of an aldehyde:

Why Does Oxymercuration-Demercuration Follow Markovnikov’s Rule?
Oxymercuration-Demercuration adds the OH group on the more substituted carbon atom of the π bond, and therefore, it is a Markovnikov addition of water to alkynes. The reason OH attacks the more substituted carbon atom is that the mercurinium ion is positively charged, and this charge is shared between the mercury and the two carbons in the ring. So, we can draw three “resonance structures” that will show that the most optimal is when the partial positive charge is on the more substituted carbon atom, because, remember, the stability of carbocation increases with the number of alkyl groups connected to the positively charged carbon atom. For example, by comparing the following structures of mercurinium ion:

As the indicated structure is the most stable, the mercurinium ion exits in this form where the more substituted carbon bears the partial positive charge, and therefore, water attacks it defining the regiochemical outcome of the reaction.
Hydroboration-Oxidation of Alkynes
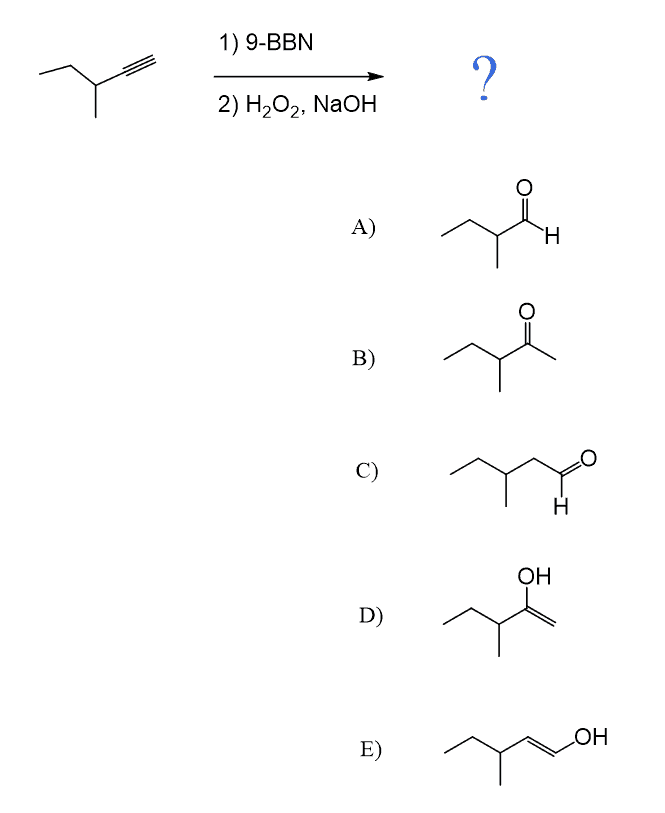
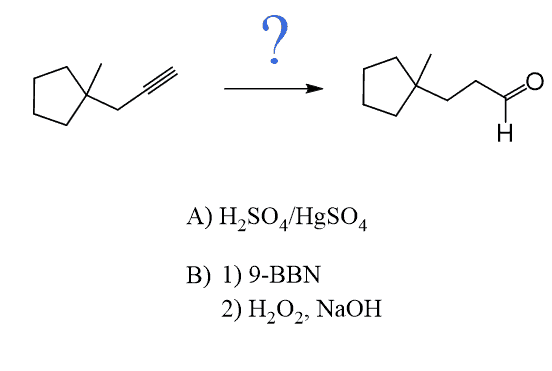
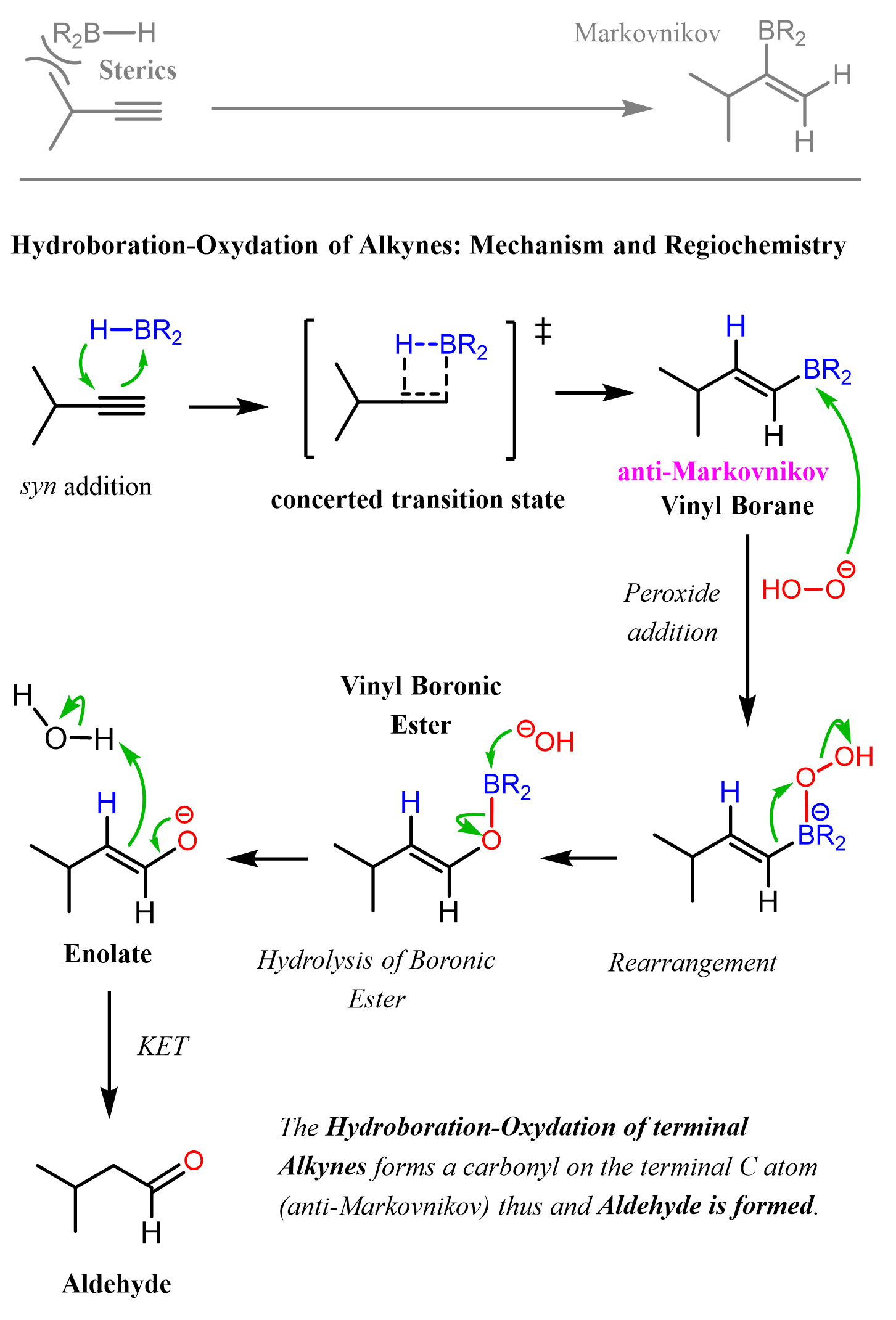
Like alkenes, alkynes can also be subjected to Hydroboration-Oxidation to achieve an anti-Markovnikov addition of an OH group to the π bond. As in the case of acid-catalyzed hydration and oxymercuration, the intermediate here is an enol which, this time, tautomerizes into an aldehyde as the OH group is on the terminal carbon atom:

Because alkynes have two π bonds, diallyl boranes (R2BH) are often used to prevent the second addition of the boron to the boron-substituted alkene. The most common alkyl borane is the 9-BBN:

The bulky nature of 9-BBN is especially handy for achieving anti-Markovnikov addition when terminal alkynes are used:

The Mechanism of Hydroboration-Oxidation of Alkynes
The reaction starts with a concerted addition of the alkylborane to the triple bond, forming a vinyl borane. Notice that this process is regioselective and due to the steric hindrance of the alkyl groups on the boron and the triple bond, which favors the opposite orientation, which adds the boron to the less substituted carbon atom (anti-Markovnikov addition). In the subsequent steps of oxidation and rearrangement, the BR2 group is replaced with an OH group, which, after a keto-enol tautomerization, forms the final product, aldehyde:
The hydroboration-oxidation of terminal alkyne gives a mixture of ketones. Depending on the difference in the bulkiness of alkyl groups, one of the ketones may form in excess.
To summarize, we learned that alkynes can be hydrated with three main methods: 1) Acid-Catalyzed Hydration, 2) Oxymercuration-Demercuration, and 3) Hydroboration-Oxidation.
The first two are used Markovnikov conversion of terminal alkynes to ketones, whereas the hydroboration-oxidation is used for anti-Markovnikov carbonylation of alkynes, where the final product is an aldehyde: