The reactivity of epoxides is due to the high ring strain of three-membered rings. The reactions of epoxides lead to the ring opening forming an alcohol with a variety of other functional groups in the product. We have seen, in the post about the reactions of epoxides, that there are two main ways the ring opening occurs:
- The ring opening of epoxides by good nucleophiles
- The ring opening of epoxide with poor nucleophiles
There are two main differences we need to remember. Good nucleophiles attack the less substituted carbon of the epoxide by SN2 mechanism forming an alkoxide ion which is protonated by acidic workup to the corresponding alcohol:

On the contrary, in reactions with poor nucleophiles, the epoxide must first be activated by protonation, and only after this, the nucleophilic attack occurs. Importantly, the addition of poor nucleophiles occurs on the more substituted carbon atom:

What is common in the ring opening of epoxides with good and poor nucleophiles is the nucleophilic attack from the opposite side of the oxygen. As mentioned earlier, this is because the ring-opening reactions of epoxide proceed by SN2 mechanism. You can check this article for more information about the regio- and stereochemistry of the ring-opening reactions of epoxides. In this post, we will narrow down to the reactions of epoxides to Grignard reagents.
The Reactions of Grignard Reagents with Epoxides
Grignard reagents are great nucleophiles and strong bases, therefore, they attack epoxides at the less substituted carbon atom. Overall, the addition of Grignard reagents to epoxides is a two-step process through which an alcohol is formed. In the first step, the epoxide ring is attacked at the least substituted carbon atom forming an alkoxide intermediate which is then treated with water or acidic workup to give the final product alcohol:
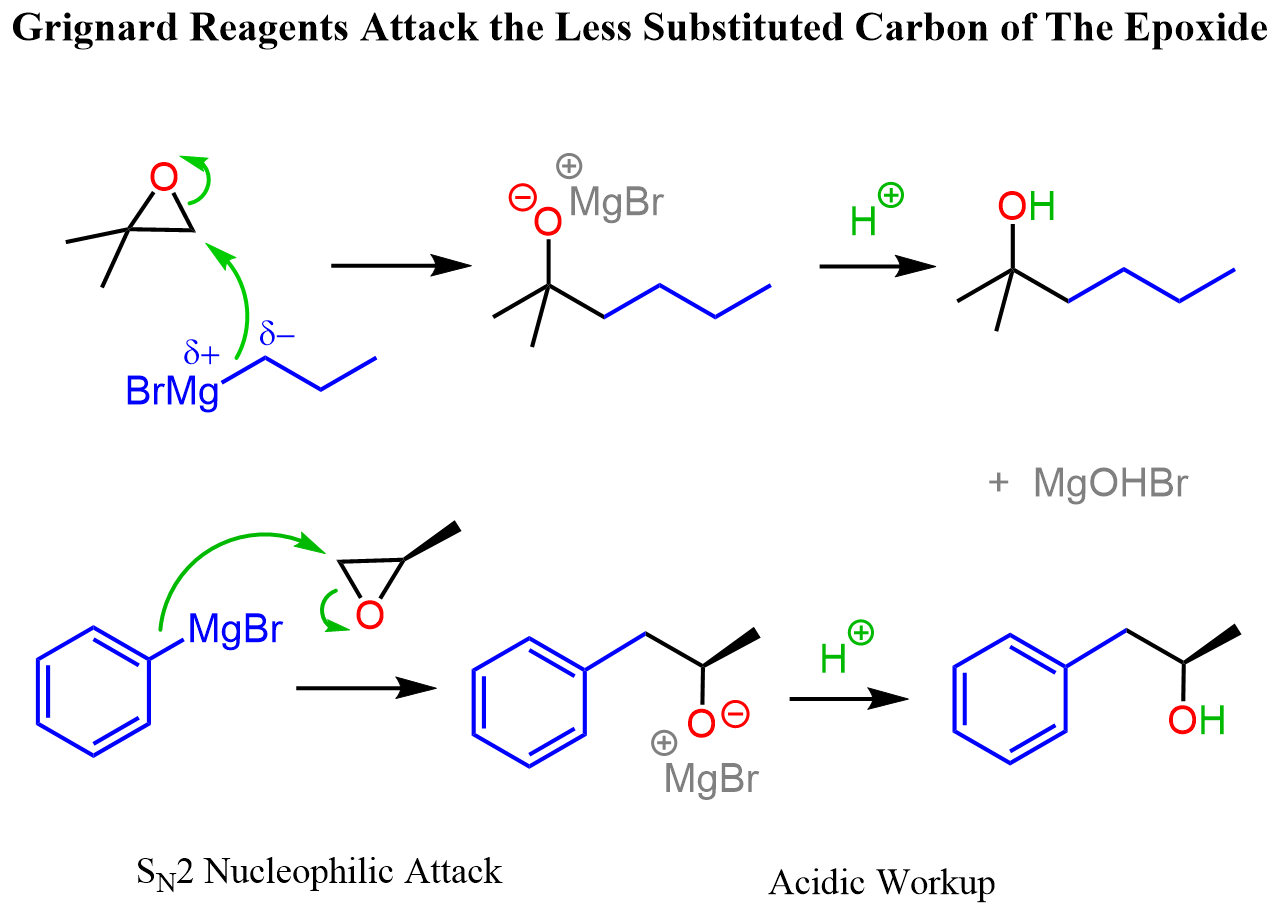
The Stereochemistry of Grignard Reaction with Epoxides
The stereochemical outcome of the reaction depends on the structure of the epoxide. Achiral epoxides most often produce achiral products. For example, the achiral epoxide in the first reaction above produces an achiral alcohol when reacted with a Grignard reagent.
The Grignard reaction of achiral epoxides can also produce a racemic mixture of enantiomers. For example, the cyclohexene epoxide is achiral as it is a meso compound. Remember, meso compounds are achiral because of the internal plane of symmetry, however, they do have chirality centers. When this epoxide is opened up with a Grignard reagent, a racemic mixture of enantiomers is obtained because breaking any of the C-O bonds breaks the symmetry of the molecule as well:

Notice that the carbon that keeps the bond with oxygen, retains the configuration, whereas the one where the nucleophilic attack occurs undergoes an inversion of configuration.
If the epoxide is chiral, each enantiomer gives the corresponding enantiomeric product. Let’s add a methyl group to one of the carbons, and see how the attack on the less substituted carbon atom produces one enantiomer of the alcohol:

This is what we had to cover for the Grignard reaction with epoxides. Check out this article on the summary of epoxide reactions as well as the practice problems to master the topic.
Epoxide Rearrangements
This is most likely not something you have covered in your class, but I wanted to add in the end of this discussion. Epoxides may have this interesting (or nice but annoying) feature of undergoing a rearrangement in the presence of Lewis acids.
For example, in the presence of LiBr and different aluminum catalysts, oxiranes have been shown to produce five-membered rings with an aldehyde group. These are types of ring contraction rearrangements:
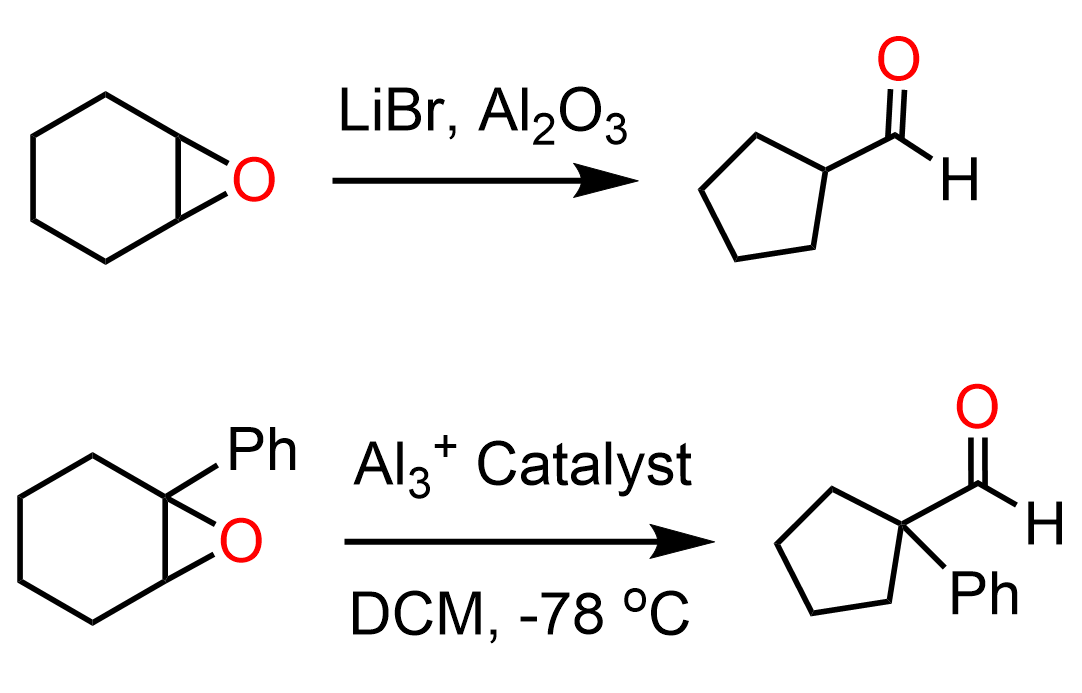
The Lewis acid catalyst activates the epoxide coordinating the oxygen. This is what we learn in the ring-opening reactions of epoxides under acidic conditions. Once the epoxide is opened, a carbocation is formed which undergoes a 1,2-ring contraction alkyl shift.
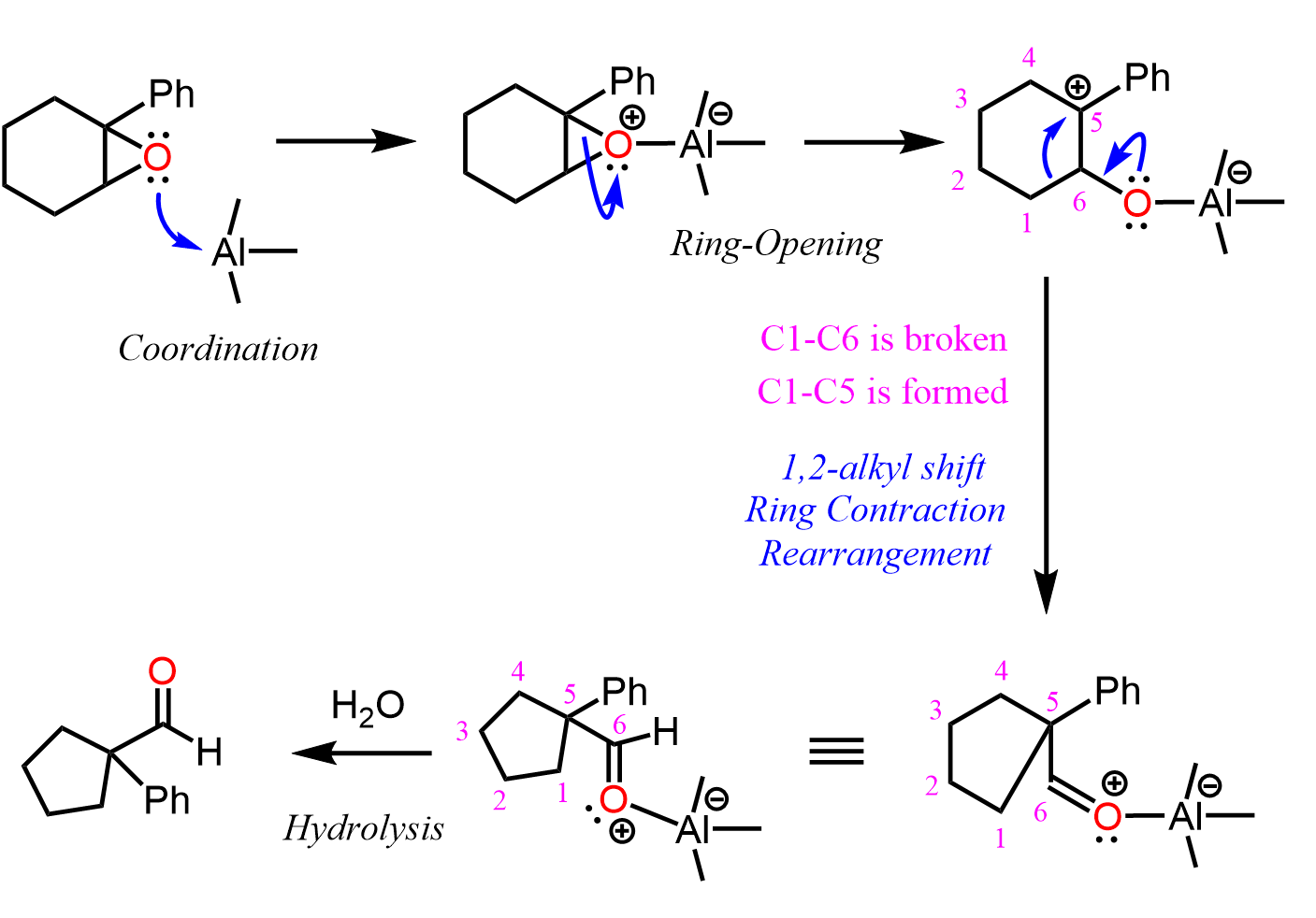
Now, instead of the aluminum catalyst, a magnesium salt such as MgBr2 can also be used to facilitate this reaction, and surprisingly or not, some Grignard reactions of epoxides have been shown to follow the same pathway (Tetrahedron Letters, 5, 1964, 1117, J. Chem. Educ. 1996, 73, 12, 1196, J. Clayden, Organic Chemistry). Here is a plausible mechanism for an epoxide rearrangement when reacted with a Grignard reagent:

The Grignard reagent is a strong base, so it is unlikely, but I have not dedicated enough time to find out whether it is the Grignard reagent itself, the residual salt, or another Lewis acid species that catalyzes this rearrangement, so if you have hands-on experience, please let us know what is actually going on here.
A nice review of ring contraction rearrangements including those of epoxides is summarized by L. Silva in Tetrahedron 58 (2002) 9137–9161.
Check Also
- Preparation of Epoxides
- Ring-Opening Reactions of Epoxides
- Reactions of Epoxides Practice Problems
- Naming Ethers
- The Williamson Ether Synthesis
- Reactions of Ethers-Ether Cleavage
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
