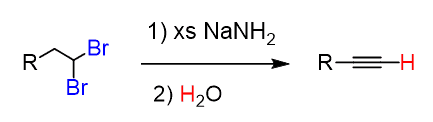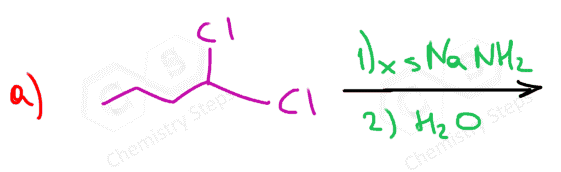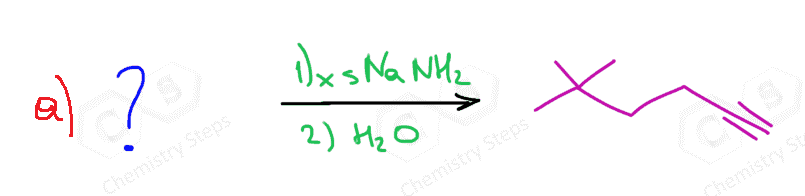Alkynes are prepared by elimination of alkyl dihalides:
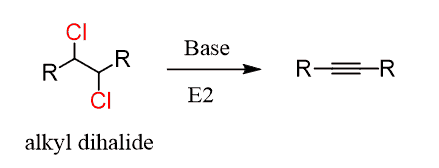
The reaction goes by E2 mechanism and is similar to how alkenes are prepared. The difference here is that two halogen atoms are needed because the triple bond id formed by two consecutive elimination reactions:

The first elimination can be performed with regular bases such as hydroxides and alkoxides. However, a stronger base is needed to achieve an elimination of the vinyl halide. Most often Sodium amide (NaNH2), dissolved in liquid ammonia (NH3), is used:

The two halogens can be on the same (geminal dihalide) carbon or on neighboring (vicinal dihalide) carbons.
To distinguish these, you can go with geminal as being “jammed” on one carbon:

More scientifically, it comes from the Latin geminus – “twin.”
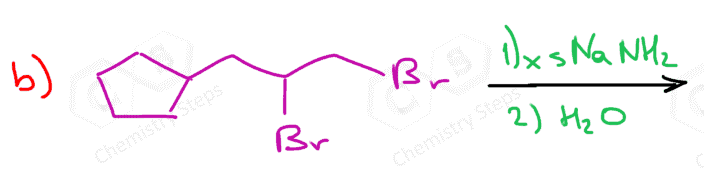
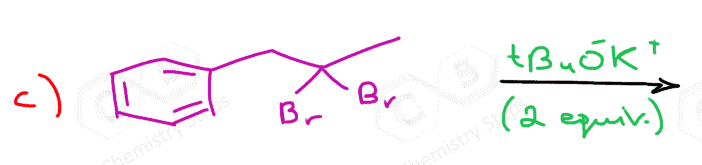
Depending on the structure of the dihaloalkane, the elimination reaction results in either an internal or terminal alkyne:

Preparation of terminal alkynes has an advantage because of the hydrogen on the triple-bonded carbon.
This hydrogen has pKa of 25 allowing for a deprotonation by the sodium amide which is the driving force for shifting the equilibrium to the right:

In total, three equivalents of sodium amide are needed: two equivalents for the two E2 eliminations and one to deprotonate the terminal alkyne.
The alkynide ion is protonated back again once the reaction is complete to regenerate the terminal alkyne. This is the work-up of the reaction and is achieved with water since the acidity of water allows to perform this acid-base reaction:
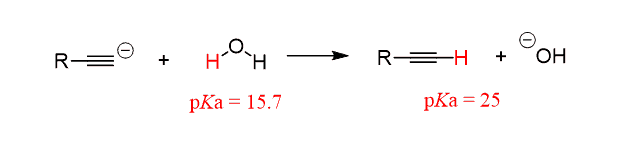
Even when an internal alkyne is prepared, an excess of sodium amide is often used to make sure the reaction goes to completion. Therefore, the water work-up is used in any case the protonate the alkynide ion and also quench the possible excess of NaNH2.
Overall, the reaction is shown like this: