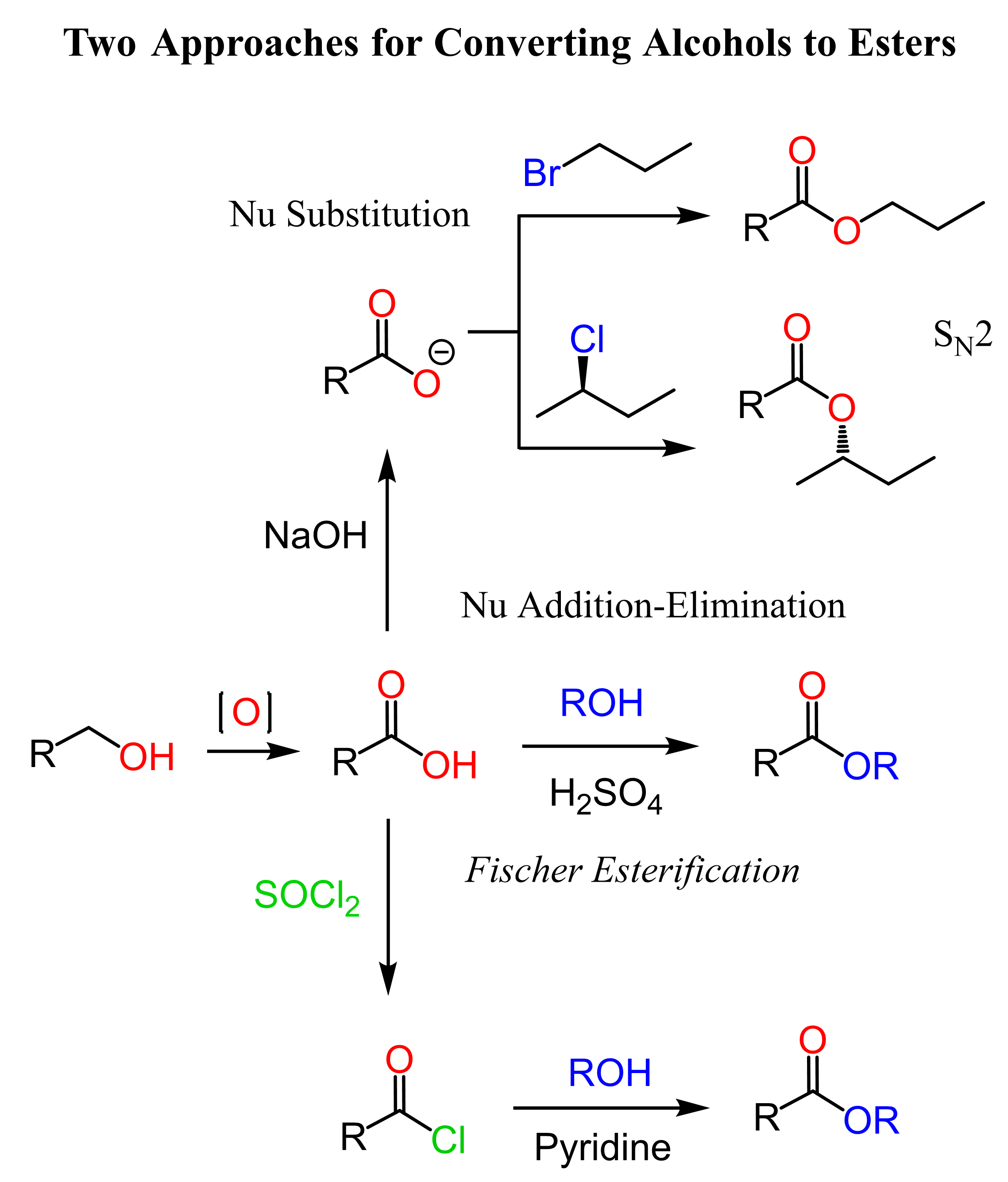
Esters are derivatives of carboxylic acids, therefore, to convert an alcohol to an ester, we need to first oxidize it to a carboxylic acid using a strong oxidizing agent such as Na2Cr2O7, CrO3, or KMnO4:

We can then react the carboxylic acid with an alcohol under acidic conditions to obtain the ester. This is known as the Fischer esterification which is a type of addition-elimination reaction of carbonyls:

We have a separate article on Fischer esterification going over the details of the mechanism, so feel free to check that out here.
In this post, we are focusing on the strategies for converting alcohols to esters. The Fischer esterification requires elevated temperatures, and if the carboxylic acid is not suitable for such conditions, we can convert it to acid chloride, which are more reactive and we can react them with esters under milder conditions:

Acid chlorides are prepared by reacting carboxylic acids with thionyl chloride (SOCl2) and other chlorinating agents:

Esters via Nucleophilic Substitution
Another strategy to convert the carboxylic acid to an ester is the use of the nucleophilicity of the carboxylate ion. Carboxylic acids are very poor nucleophiles, however, if we deprotonate them, the resulting carboxylate ions are quite nucleophilic and we can use them for SN2 substitution with alkyl halides:

In the end, let’s summarize the strategies for converting primary alcohols to esters in one synthetic scheme:
Secondary Alcohols to Esters
We have seen how primary alcohols can be incorporated to be the alkyl component of the ester. The same strategy such as Fischer esterification, reacting them with acid chlorides would still work for secondary alcohols.
Now, what about if need to convert the secondary alcohol to the acyl component of an ester?
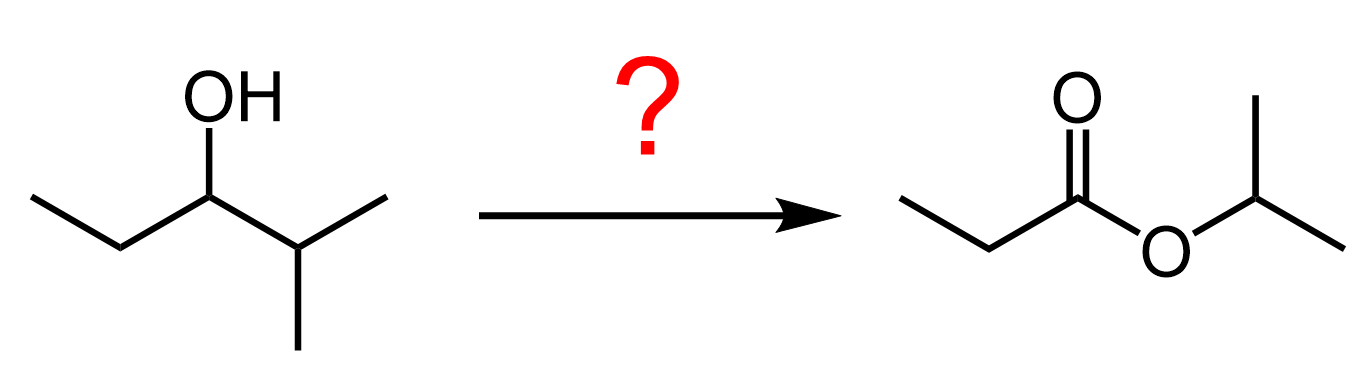
There are a few strategies for this including the Baeyer-Villiger Oxidation, and we need to first oxidize the alcohol to a ketone. Recall that Baeyer-Villiger Oxidation is a rearrangement where the oxygen atom is inserted between the carbonyl group and the more substituted alkyl group.
For example, we can convert ethyl isopropyl ketone (2-methyl-pentan-3-one) to isopropyl propionate:
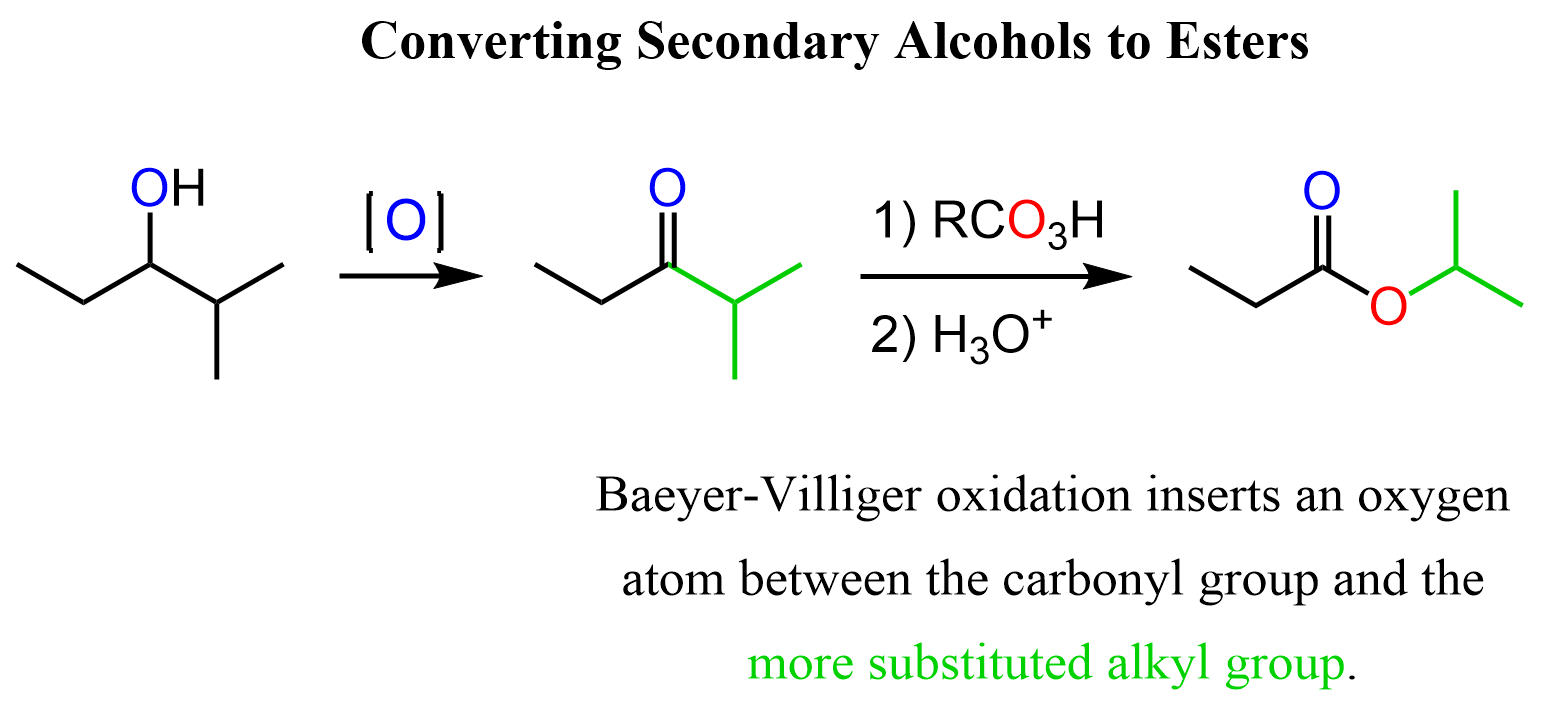
You can find the mechanism of Baeyer-Villiger Oxidation in this post on the conversion of aldehydes and ketones to carboxylic acids.
If we need to introduce another alkyl group to the ester, we can dehydrate the alcohol to the Zaitsev or Hofmann alkene, cleave the double bond of the alkene to a carboxylic acid, and finally introduce the needed alkyl component:

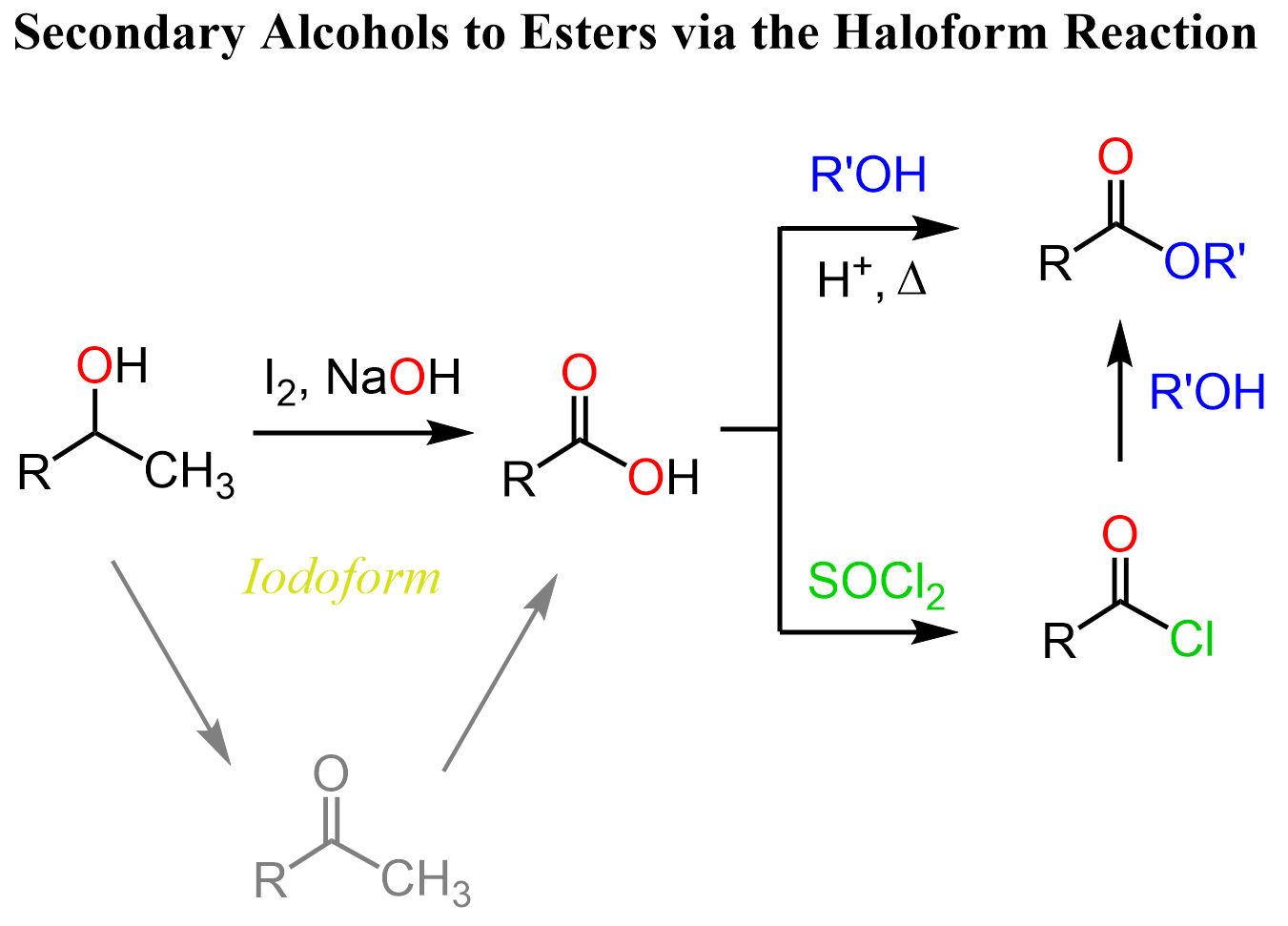
The Haloform Reaction of Secondary Alcohols
If we have a methyl secondary alcohol, we can also use the haloform reaction to convert it into a carboxylic acid. The Haloform reaction is mostly known for the conversion of methyl ketones to carboxylic acids but methyl alcohols are oxidizing to the corresponding carbonyl, therefore, they can also be used for this purpose:
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- The reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz

