In this post, we will discuss the reduction of carboxylic acids and their derivatives, such as acid chlorides, esters, amides, and nitriles. Let’s start with carboxylic acids.
Reduction of Carboxylic Acids
Carboxylic acids can be converted to alcohols using an excess of a strong reducing agent such as LiAlH4.

Aside from the acidic workup in the last step, the reduction is overall a three-step conversation. Remember that LIAlH4 is a strong base, so the first step is the deprotonation of the carboxylic acid. In the next two steps, we have a nucleophilic addition of the hydride to the carbonyl group; thus an excess of reducing agent is needed:

It is worth mentioning that NaBH4 is not effective in reducing carboxylic acids and esters, because the intermediate carboxylate ion is not as electrophilic, and the addition of hydride requires a more electron-rich reducing agent. The hydride addition is facilitated also by a coordination of the Al to the carbonyl oxygen. This withdraws some of the electron density, thus making the carbon more electron-deficient. After the hydride addition, we have an elimination step where the –OAlH2 is expelled, resorting the carbonyl in the form of an aldehyde:

Notice that an aldehyde is another intermediate in this reaction, but we cannot stop the reduction at this stage. Remember, aldehydes are more reactive than carboxylic acids, esters, and especially carboxylate ions because there is no resonance donation of the oxygen to the carbonyl carbon, which reduces the electron-deficient character of the carbonyl.

The greater reactivity of the aldehyde explains why it cannot be isolated as the final product.
Reduction of Carboxylic Acids with Borane
We saw in the mechanism above that the carbonyl is activated by the coordination of the oxygen to Al, which serves as a Lewis acid there. Now, borane is another Lewis acid, and when coordinated to an oxygen, it becomes electron-rich and thus a hydride donor. This turns out to be a good tool for reducing carboxylic acids to alcohol. It is still an addition-elimination reaction to the carbonyl, however, the intermediate is not an aldehyde by rather a boronic ester:
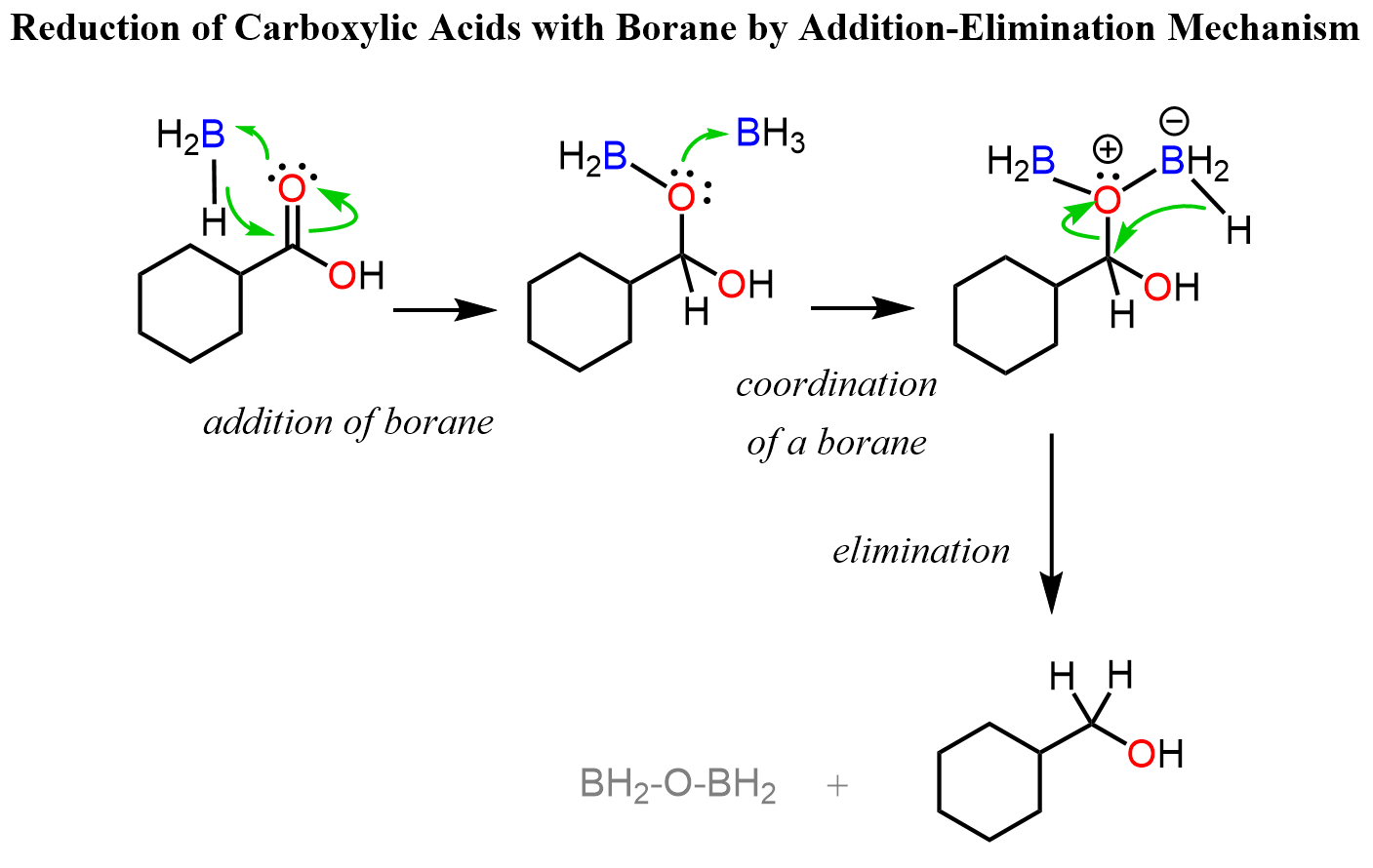
You can read more about it in different articles including https://doi.org/10.1016/j.jphotobiol.2015.04.015.
Reduction of Esters
Like carboxylic acids, esters also require strong reducing agents to convert them to alcohols. What is different is that the alkyl group plays the role of a protecting group, and esters do not immediately undergo an acid-base reaction with the reducing agent. Because of this, esters can also be reacted with Grignard or organolithium reagents. This is not a reduction in the conventional meaning of this word, but rather a synthetic transformation. However, the carbonyl carbon is still reduced, as we’ll see later in this section.
Reduction of Esters to Alcohols using LiAlH4
Like in the case of carboxylic acids, we need an excess of LiAlH4 to convert esters to primary alcohols:

The mechanism is similar to the reduction of carboxylic acids, and we still have the aldehyde as a more reactive intermediate.

Reduction of Esters to Aldehydes by DIBAL
As mentioned earlier, the reduction of esters with LiAlH4 cannot be stopped at the aldehyde because of its greater reactivity. Now, if the intermediate is more reactive, perhaps we could use a reducing agent that is less reactive. This is the idea behind using a bulky reducing agent, Diisobutylaluminium hydride, also known as (DIBALH, DIBAL, DIBAL-H or DIBAH). The reaction is carried out at lower temperatures (typically -78 oC as that is the temperature of dry ice):
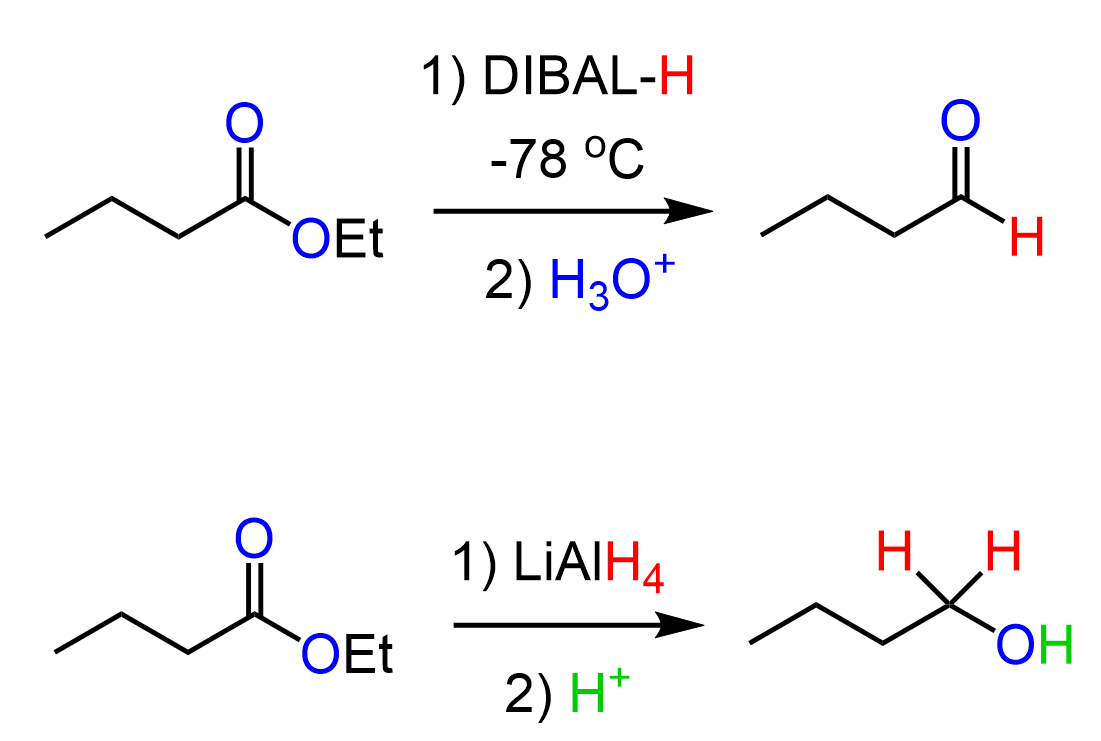
The mechanism is similar to the reduction with LiAlH4, however, notice that the intermediate, formed after the addition of the hydride, is very bulky, and the second addition does not occur as readily. So, with the correct temperature and good timing, we can quench the reaction by an aqueous, then acidic workup, which also hydrolyzes the bulky intermediate to an aldehyde:
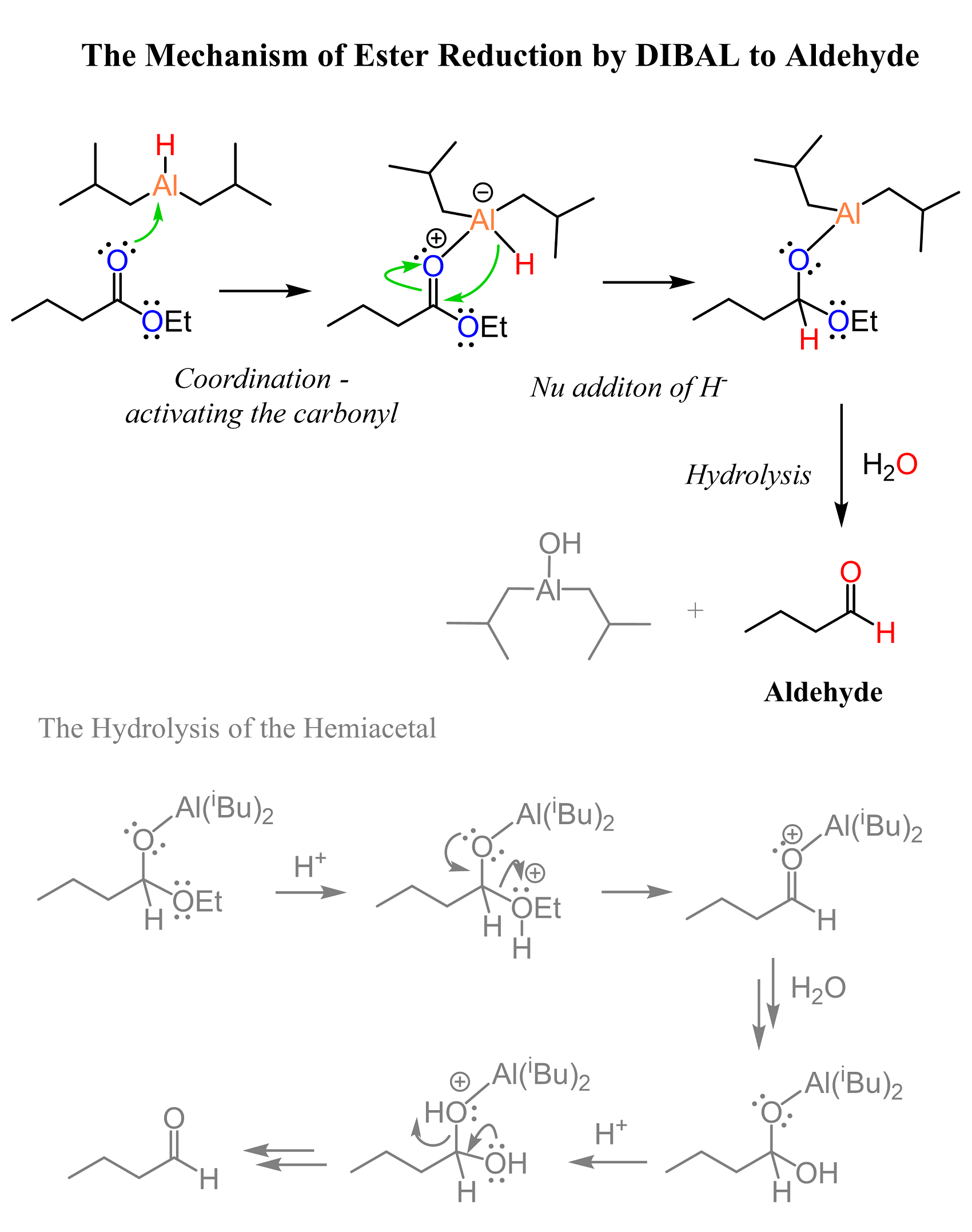
This mechanism is what we saw in the hydrolysis of hemiacetals, so feel free to check it for a better comparison.
Reducing Esters to Tertiary Alcohols with Organometallics
Esters can be converted to tertiary alcohols by reacting them with two equivalents of a Grignard reagent:
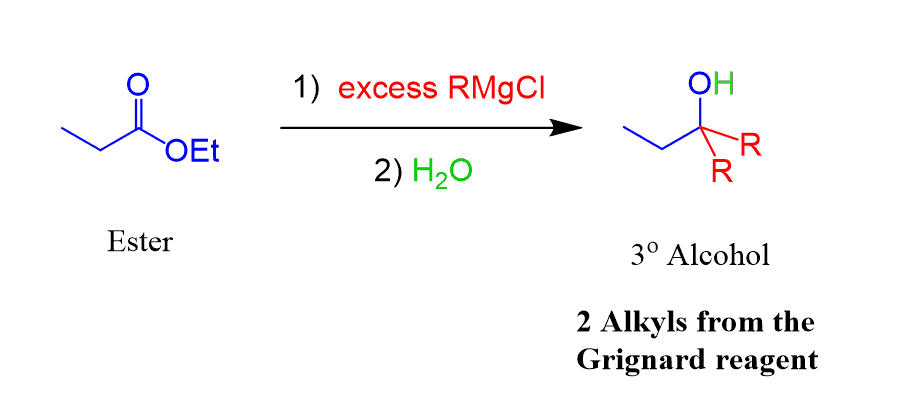
In the first step, we have the nucleophilic attack of the Grignard making the C-C bond and shifting the electrons of the π bond to the oxygen. The second equivalent is needed because the product of the first addition-elimination reaction to the carbonyl is a ketone (was an aldehyde in the case of LiAlH4), which is now converted into a tertiary alcohol:

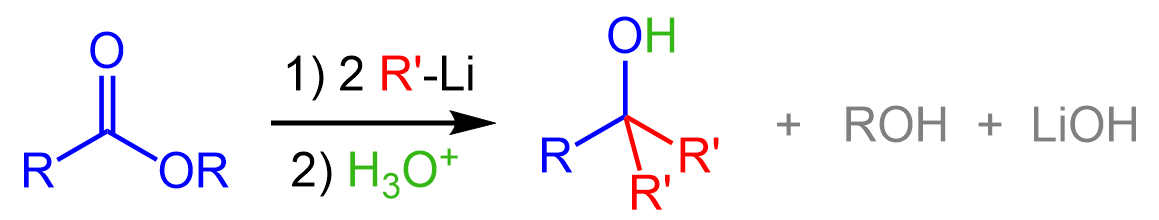
Similarly, organolithiums will also convert an ester to a tertiary alcohol:
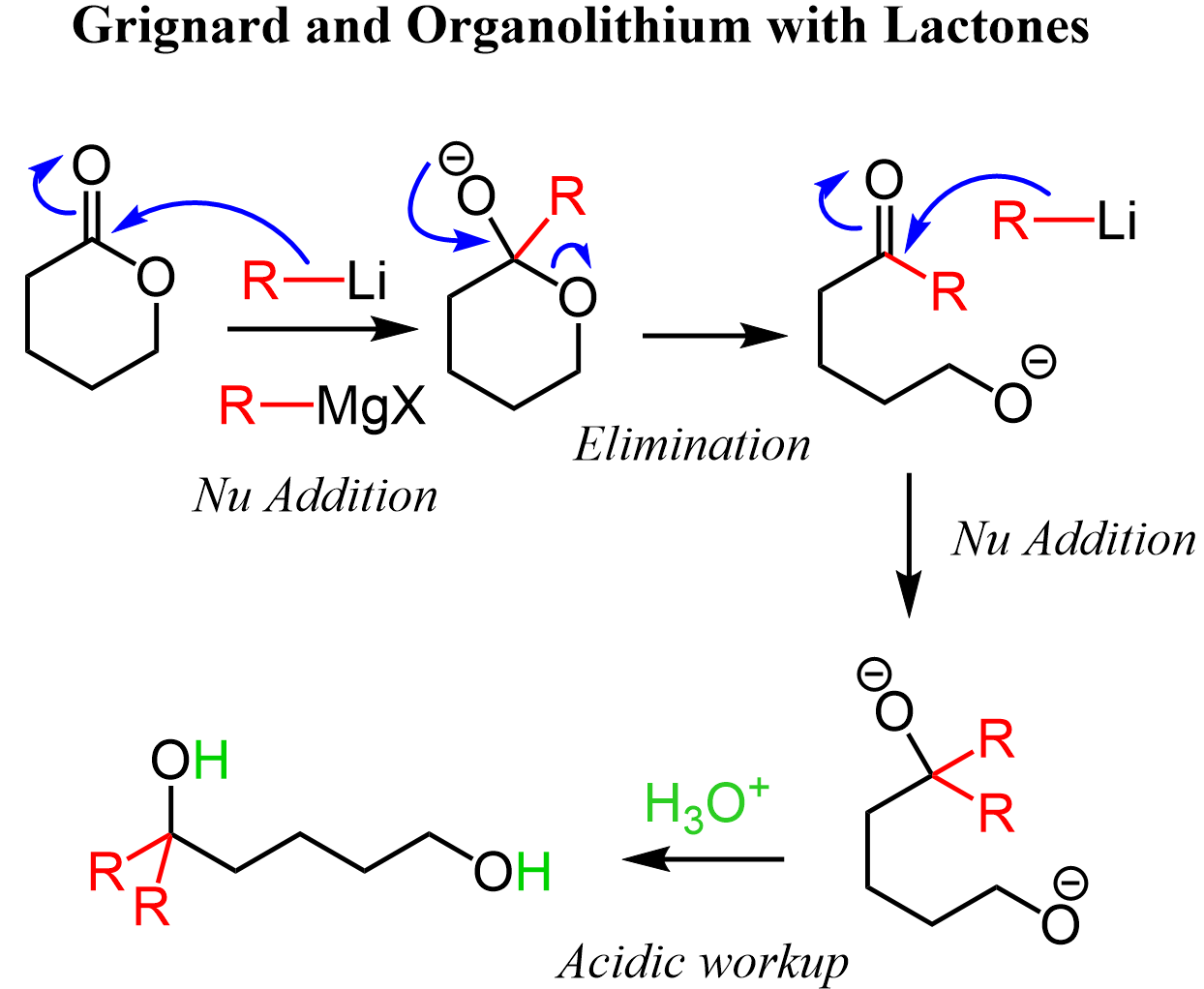
Let’s also show the mechanism of an organolithium with cyclic esters known as lactones. The steps are the same: we have a nucleophilic addition-elimination which forms a ketone and opens the ring. The ketone is then attacked by the carbon nucleophile, and after the workup, two alcohol groups are formed:
Reduction of Acid (Acyl) Chlorides
Acid chlorides are the most reactive among the carboxylic acid derivatives, and therefore, they can be reduced to alcohols by lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4).
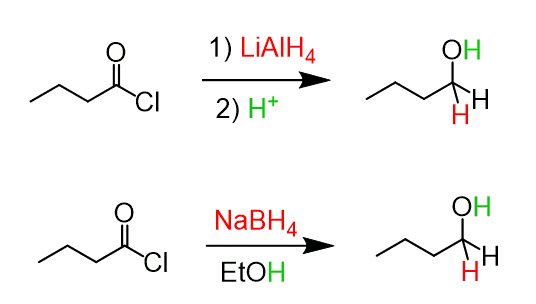
Like in the case of acids and esters, an aldehyde is formed here as an intermediate, so to force the reaction to completion, an excess of the reducing agent is used:
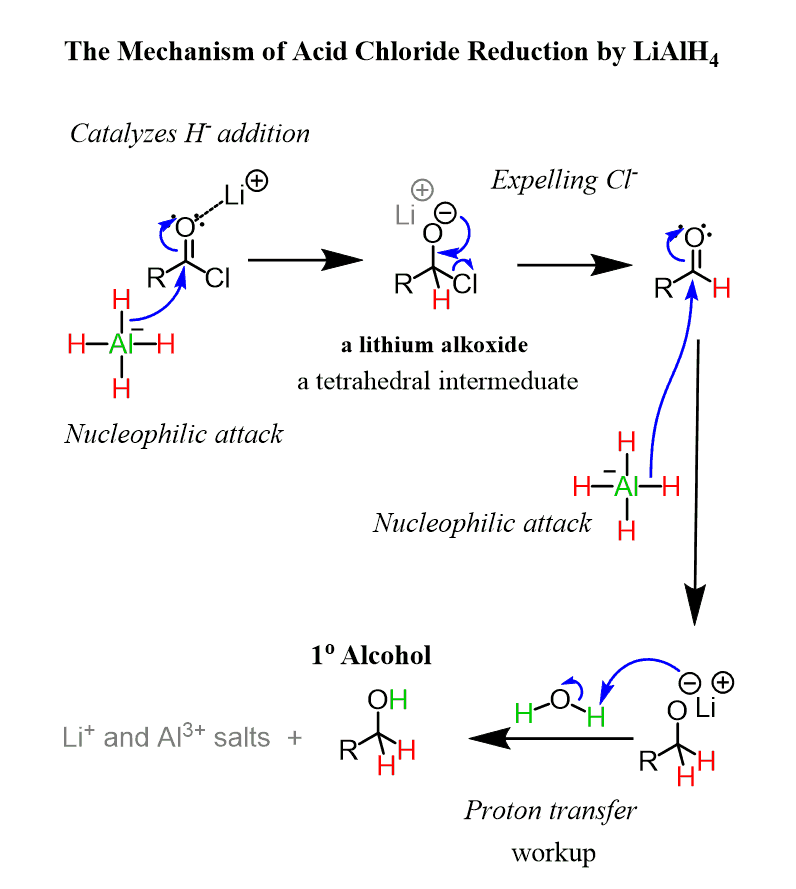
Reduction of Acyl Chlorides to Aldehydes
We have seen that esters can be reduced to aldehydes using a bulky reducing agent DIBAL as it makes the second addition to the carbonyl more sterically challenging. The same strategy is used in the case of acid chlorides, which can be reduced to aldehydes by using LiAl(OtBu)3H (Lithium tri(t-butoxy) aluminum hydride):
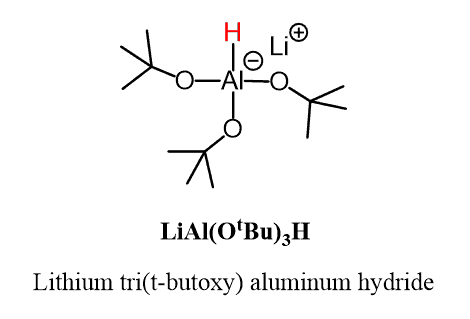
Lithium tri(t-butoxy) aluminum hydride LiAl(OtBu)3H is a derivative of LiAlH4, where three of the four hydrogens are replaced with tBuO groups. This modification reduces the reactivity of the hydride ion, and although it still rapidly reacts with the acid chloride, the slower reaction with the aldehyde allows it to be isolated from the reaction mixture:

The best yields are obtained when the reaction is carried out at -78oC (sublimation point of dry ice).
Reduction of Nitriles
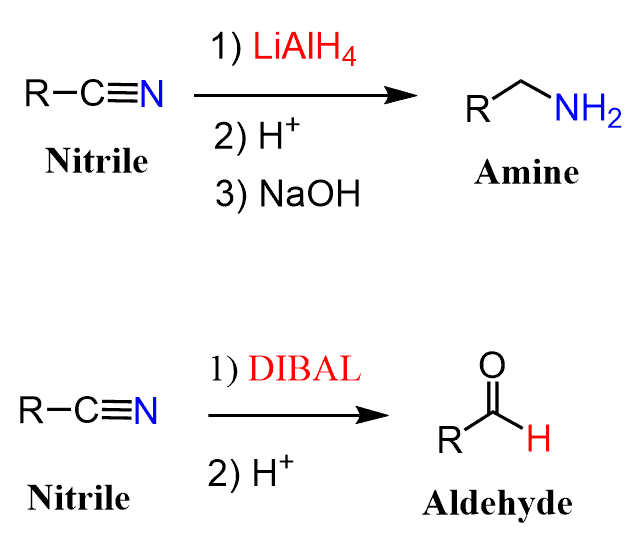
Like esters, nitriles can be reduced by LiAlH4 or DIBAL to primary amines and aldehydes, respectively:
The Mechanism of Nitrile Reduction with LiAlH4
The reaction starts with a nucleophilic addition of the hydride ion. This forms an imine salt, which undergoes another nucleophilic addition by AlH3, producing a highly reactive derivative of an amine. Both N-Al and N-Li bonds in this derivative are very polar and quickly react with water, forming the new N-H bonds of the primary amine:

Notice that the arrow starting from the N-Li bond indicates the electrons forming an N-H bond and not Li-H. The lithium is almost ionic (positively charged) and reacts with the -OH forming LiOH. Likewise, in the second step of the workup, the arrow starting from the N-Al bond forms a new N-H bond and Al(OH)H2.
The Mechanism of Nitrile Reduction by DIBAL
The reduction occurs by a coordination of the nitrogen to the aluminum, thus activating the triple which allows for the addition of the hydride. Like in the reduction of esters to aldehydes using DIBAL, there is also a bulky intermediate formed after the addition of hydride to the unsaturated carbon of the nitrile. The bulkiness of the reducing agent and the iminium salt intermediate prevents the second hydride addition, and the salt is hydrolyzed to the corresponding aldehyde:

I will use the hydrolysis of the iminium salt as an exercise to work on, and you can use the example of ester reduction and the mechanisms of hydrolyzing imines to aldehydes to do it.
In the end, I want to mention that all of these, but especially the reduction with DIBAL to aldehydes, are a lot easier on paper than in the lab. Sometimes, it is more practical to reduce the derivative completely and oxidize it back to an aldehyde or find alternative approaches to achieve the desired transformation.