Let’s discuss a few ways of converting ketones to amides, starting with the Beckmann rearrangement. It is not the ketone that undergoes a rearrangement but rather the intermediate oxime:
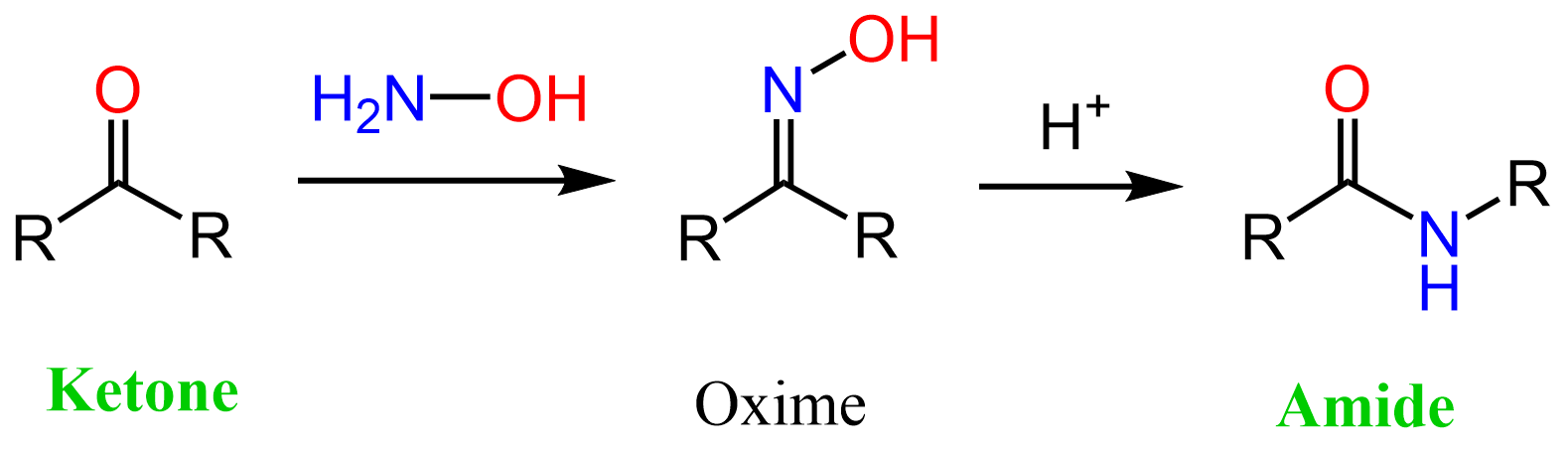
Oximes are prepared from the ketone under acidic conditions, as we saw for the imines. The idea, or maybe it is more accurate to say the reason, behind the Beckmann rearrangement is that one of the alkyl groups of the ketone jumps to the nitrogen kicking out the protonated OH group:
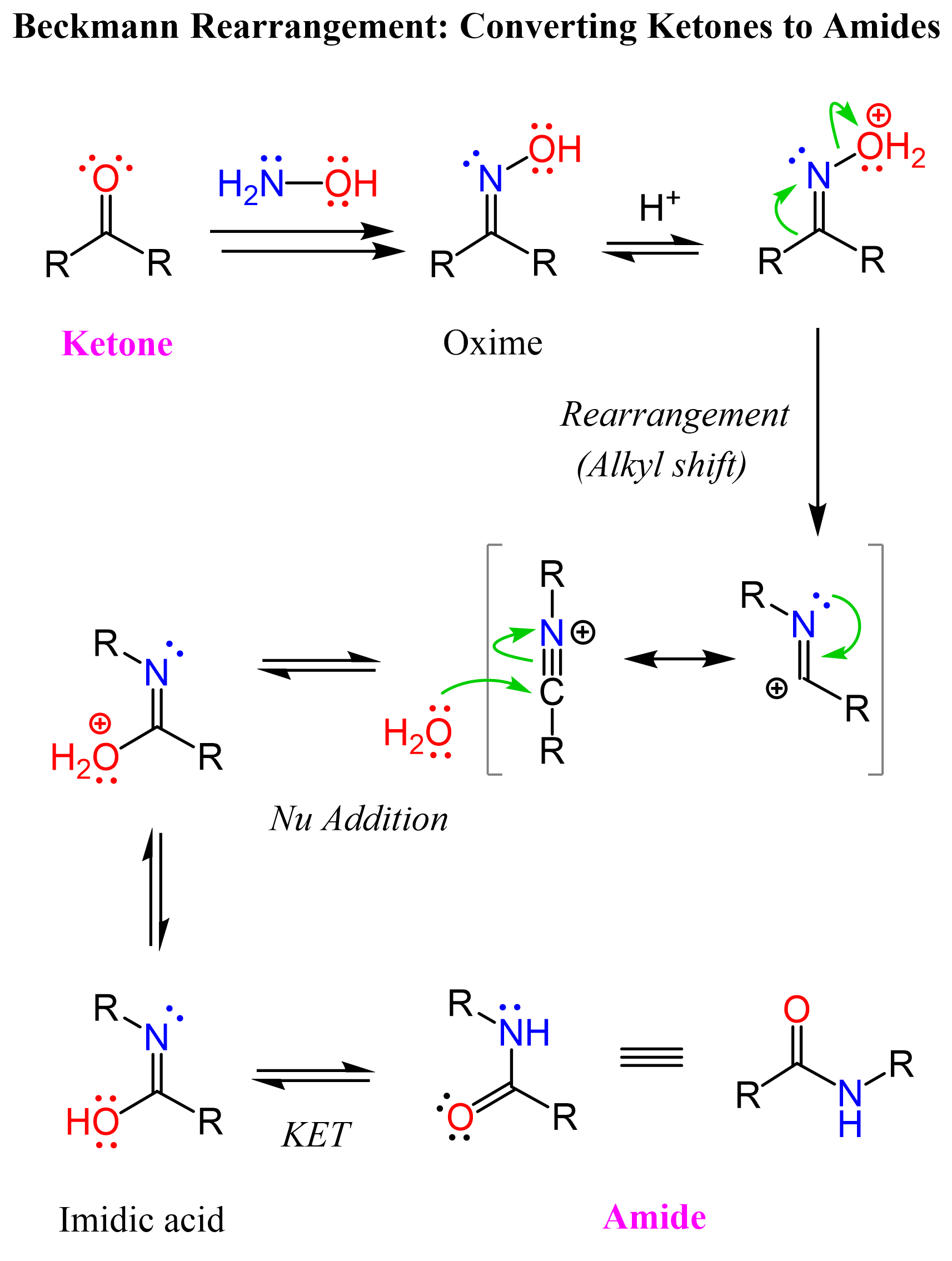
As a result of the alkyl shift, an iminium ion is formed, and, being very electrophilic, it is attacked by water, forming imidic acid, which tautomerizes to the corresponding amide.
Ketones to Amides via Carboxylic Acids
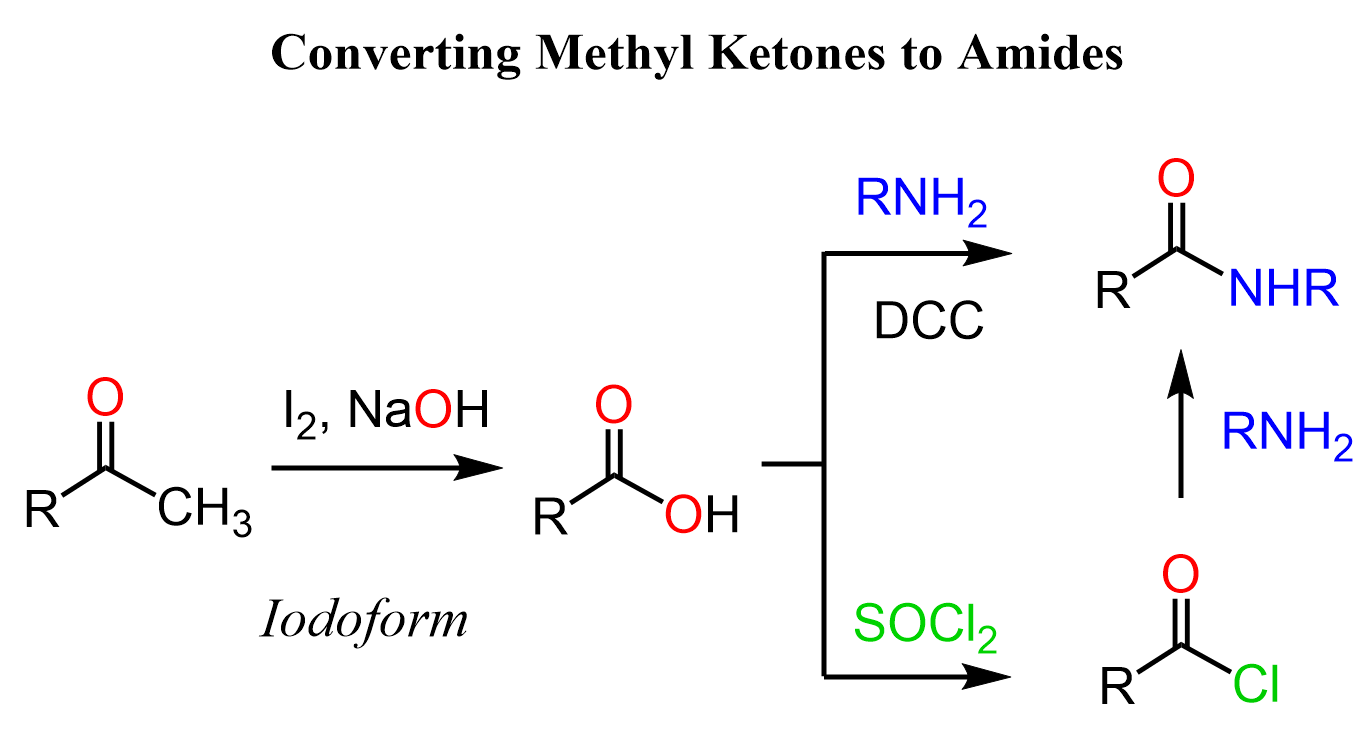
Amides are derivatives of carboxylic acids, therefore, another strategy to convert ketones to amides is by first converting them to carboxylic acids. For methyl ketones, we can use the haloform reaction. When methyl ketones are halogenated, an intermediate trihalogenated ketone is formed. The addition of hydroxide ion and subsequent expulsion of the well-stabilized -CX3 ion gives the needed carboxylic acid. The reaction is most known as iodoform (CHI3) because it is a bright yellow solid serving as an indicator for methyl ketones being oxidized to carboxylic acids in the presence of iodine:

Once we have the carboxylic acid, we can subject it to condensation with amines using a coupling agent such as DCC or EDC. Alternatively, we can convert the carboxylic acid to acid chloride, which are the most reactive carboxylic acid derivatives, and readily react with all types of nucleophiles including amines:
Ketones to Amides via Oxidative Cleavage
Let’s also mention one approach that, although lengthy, maybe a great way of converting ketones to amides. Well, at least on paper, I should say.
We first convert the ketone to an alkene via a reduction and either Zaitsev or Hofmann elimination, and oxidize the alkene to a carboxylic acid by either ozonolysis or KMnO4. Once we have the carboxylic acid, we can condensate it with an amine using a coupling reagent or via converting it to an acid chloride first:
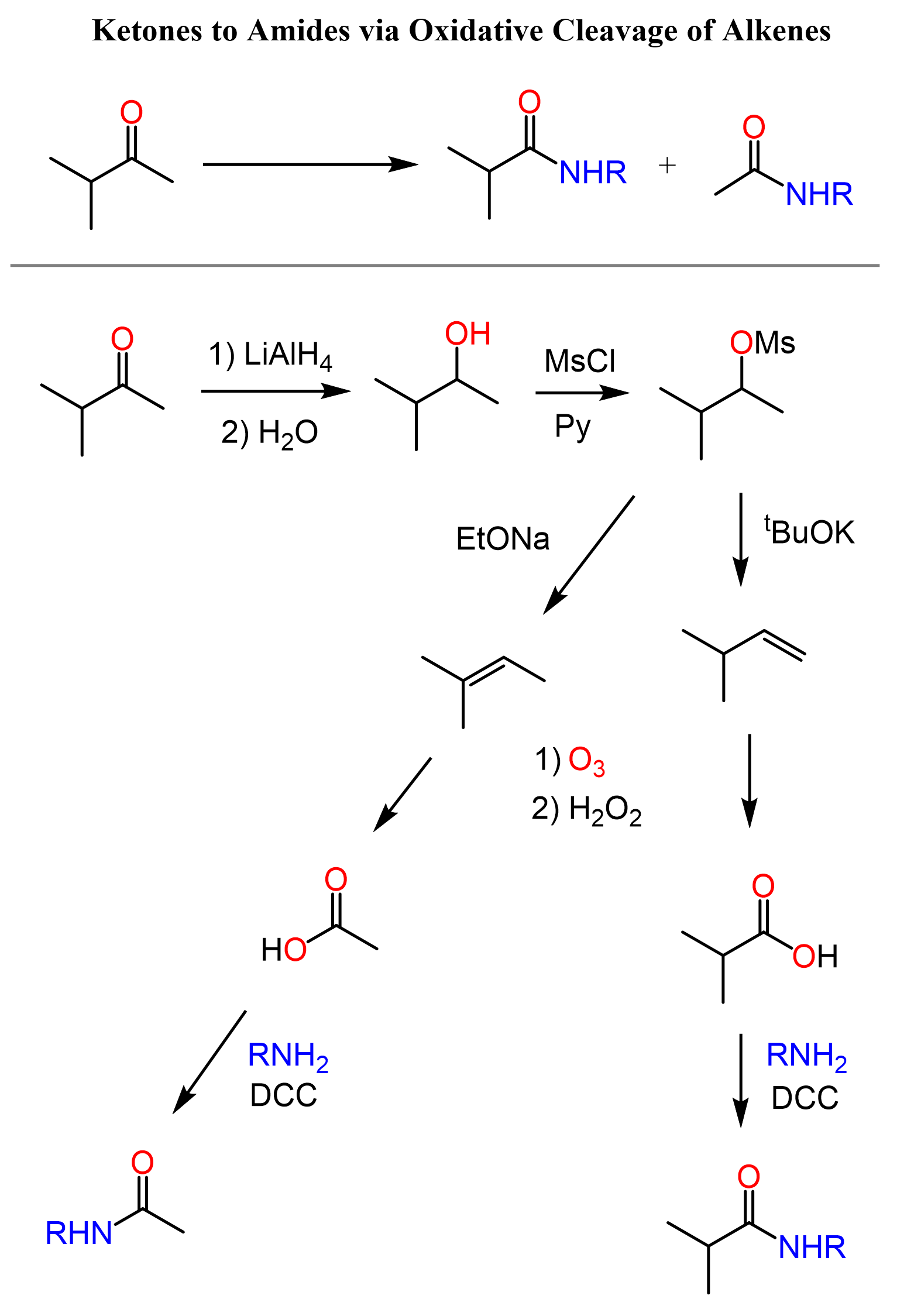
So, what is great about this method, you may ask? Notice that we have an option of selectively replacing one of the alkyl groups with the amino part of the amide. In addition, it is always good to have a spare plan if the other approaches do not work. For example, the haloform reaction is restricted to methyl ketones, and we don’t want to rely solely on the Beckmann rearrangement.
As always, please let me know in the comments if you have other methods in mind to convert ketones to amides.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Nomenclature of Aldehydes and Ketones
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- The Addition-Elimination Mechanism
- Reduction of Carbonyl Compounds by Hydride Ion
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- Reactions of Aldehydes and Ketones with Amines-Practice Problems
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Reaction of Aldehydes and Ketones with CN Cyanohydrin Formation
- Hydrolysis of Acetals, Imines and Enamines-Practice Problems
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction-Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz

