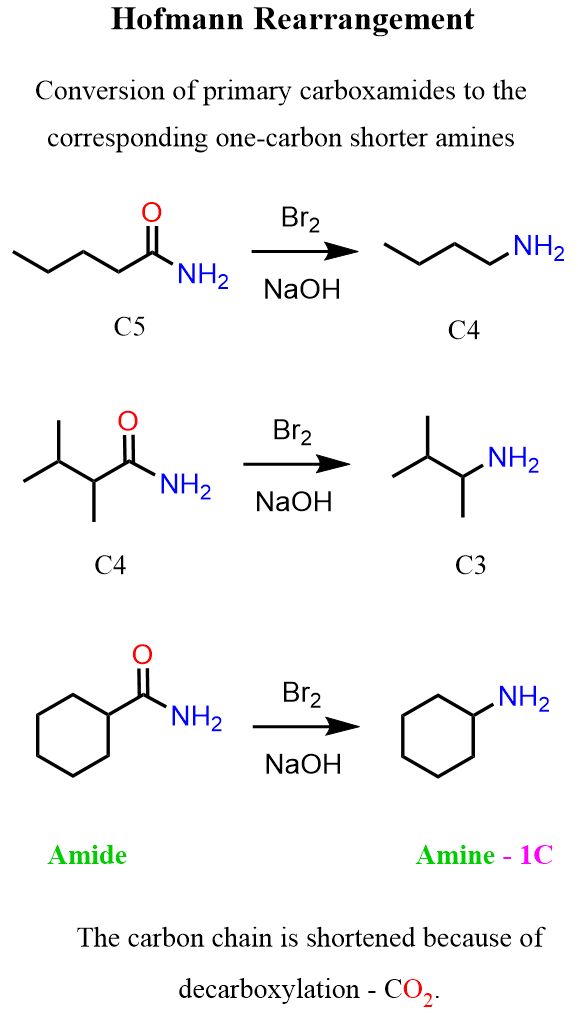
In this post, we’ll talk about the Hofmann rearrangement, a reaction that’s used to convert primary amides into primary amines.
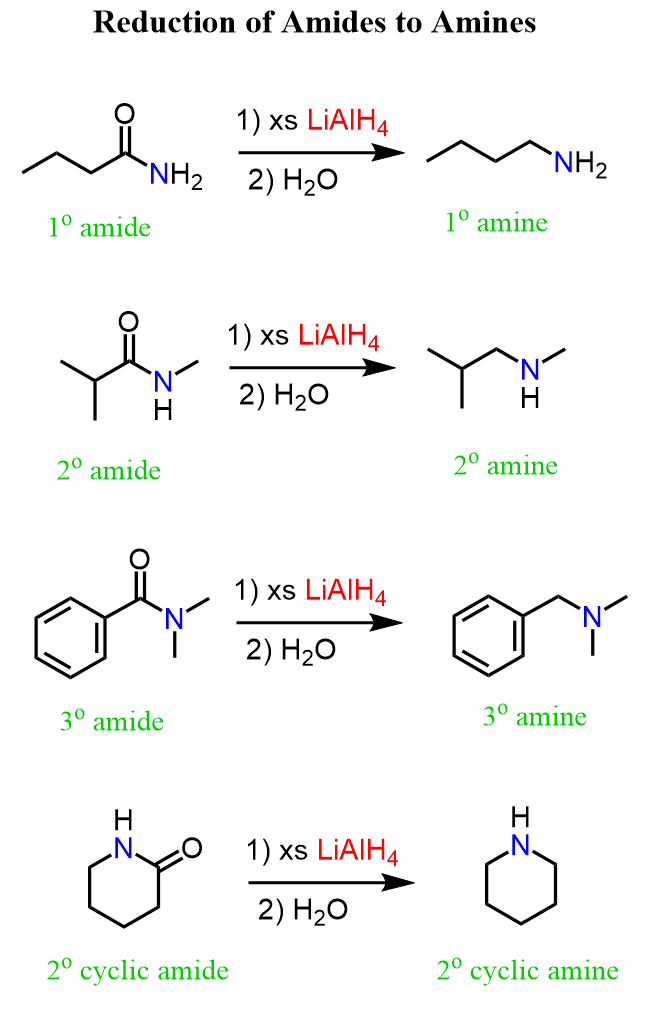
We know from earlier discussions that amides are generally converted to amines by reducing them with LiAlH4.
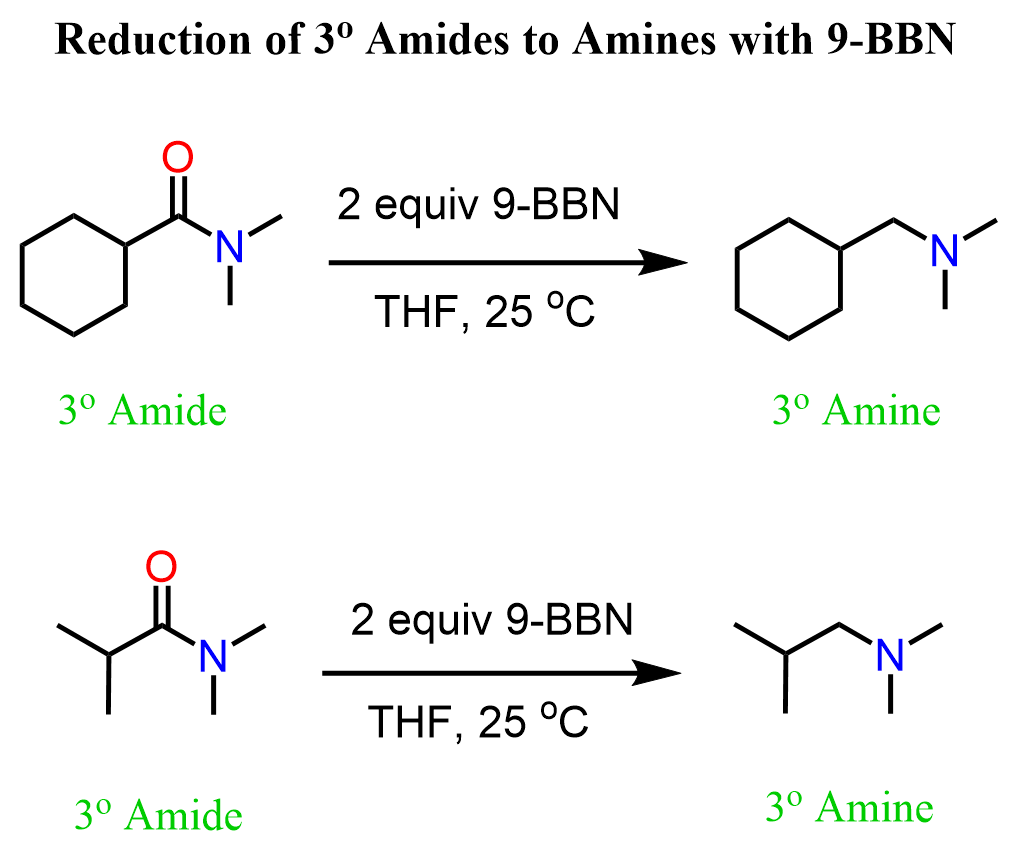
A less common strategy is the use of 9-BBN, which we know from the hydroboration-oxidation reactions. This method is limited to tertiary amides, and we have a detailed post that also covers the mechanism, so feel free to check that out.
So, what is the difference/advantage of the Hofmann rearrangement compared to the reduction of amides? Well, the first thing is to notice that the product has one fewer carbon than the starting material. This is due to the decarboxylation that happens in the last part of the conversion. Second, there may be times when you want to avoid using reducing agents because of other functional groups present in the molecule, and last but not the least, it is simply another method, so why not use it?!
The Mechanism of Hofmann Rearrangement
There are two main parts to the Hofmann Rearrangement: 1) The formation of electrophilic intermediate Isocyanate – the magic step here is the 1,2-shift of the alkyl group connected to the carbonyl carbon, 2) The hydrolysis of the isocyanate, which leads to decarboxylation, thus giving the final product, amine.
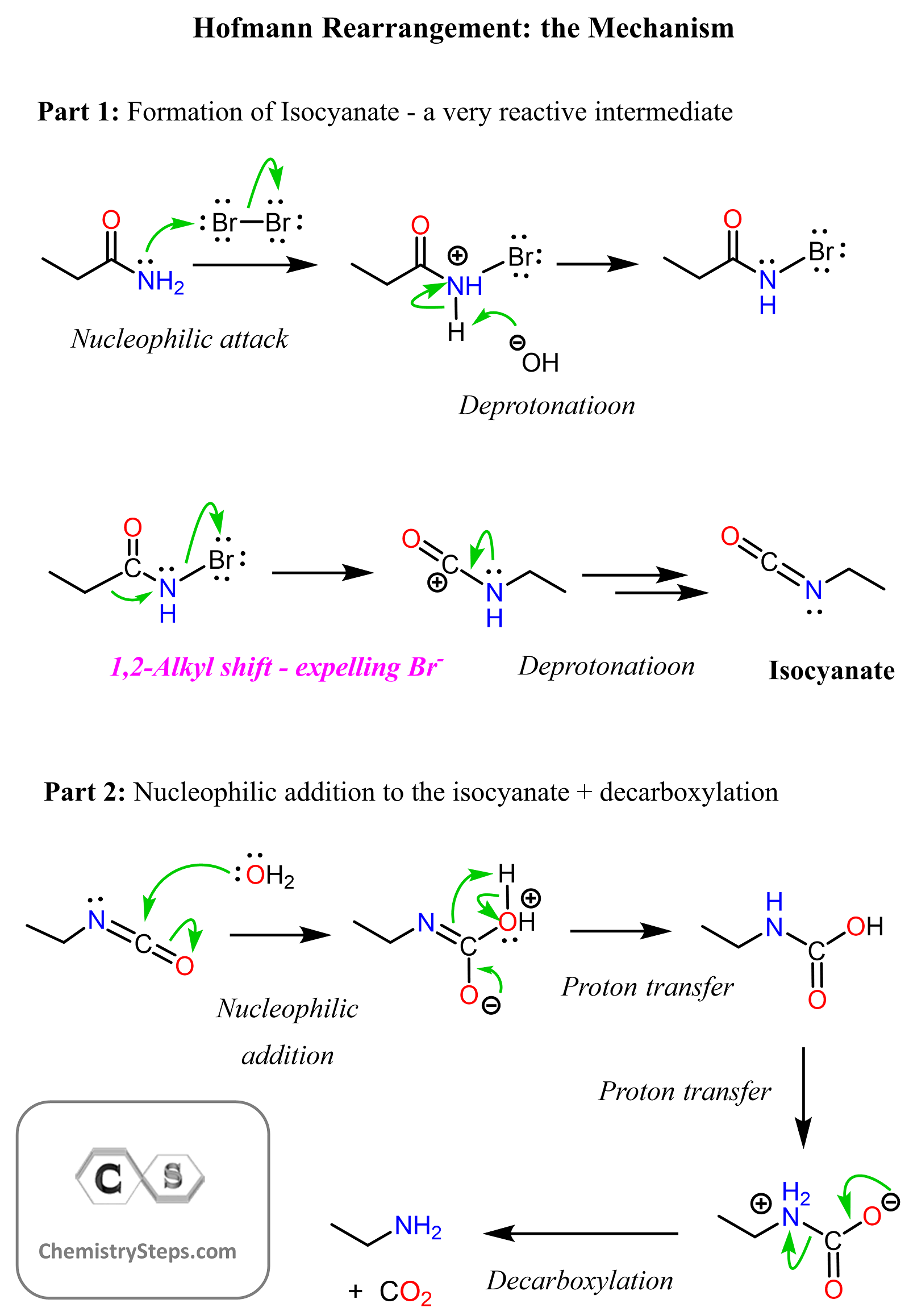
Notice that the role of bromine is to serve as a leaving group and facilitate the 1,2-shift of the alkyl group, forming the intermediate isocyanate. The isocyanate is very electrophilic and thus immediately attacked by water, forming another intermediate, carbamic acid. The decarboxylation of the carbamic acids gives the amine as the final product. Recall that we have also seen this in the removal of Boc protection group for amines.
The Hofmann rearrangement works for alkyl and aryl amides, but they must be primary amides. This is what I wanted to share about the Hofmann reaction, so feel free to ask in the comments if you have any questions.
Check Also:
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
