There are a few ways to convert nitriles to esters. Let’s start with the Pinner reaction, which, although not commonly highlighted in textbooks, is a direct and efficient method for this transformation.
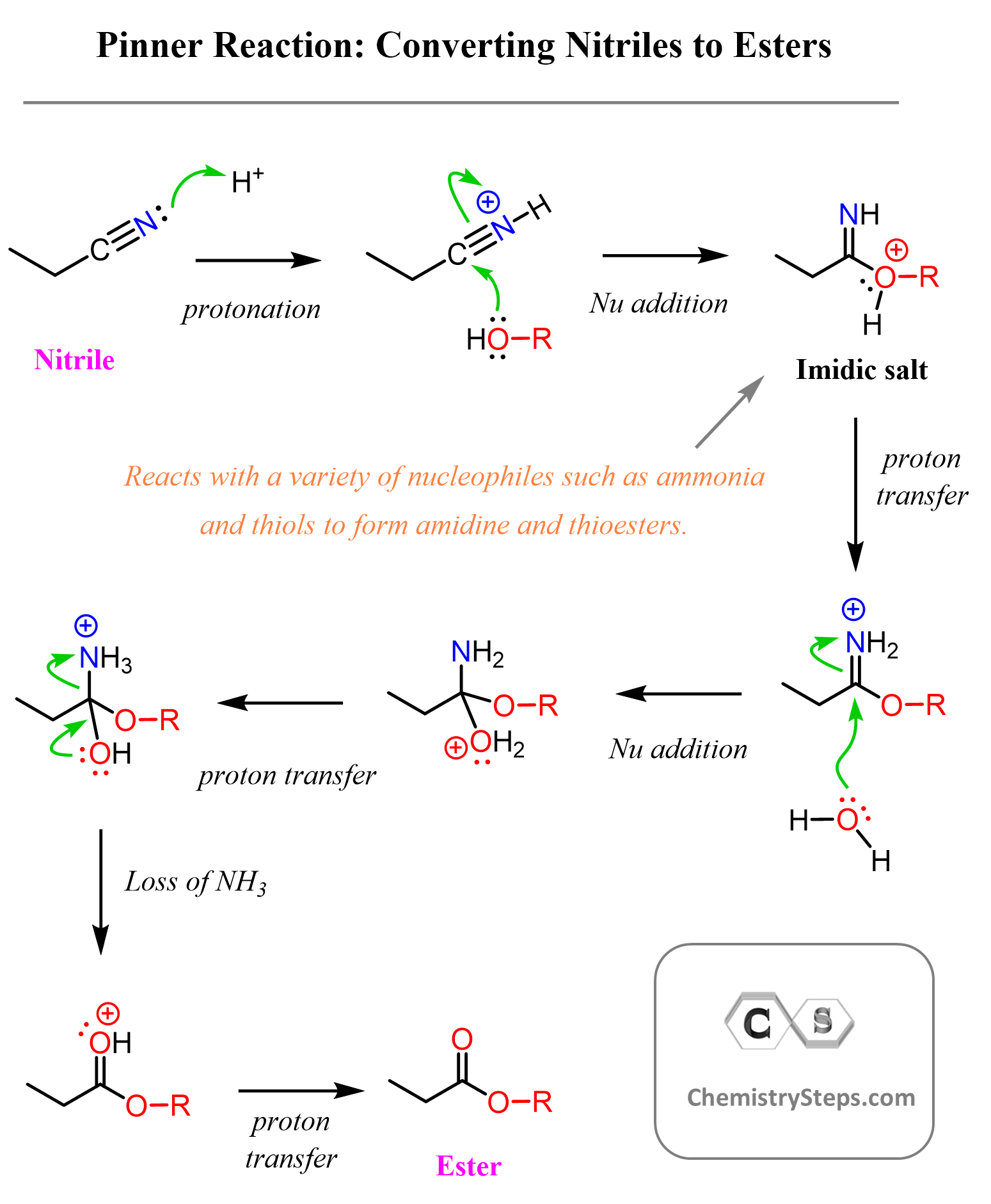
Pinner Reaction for Converting Nitriles to Esters
The reaction starts with protonation of the nitrogen, thus making the carbon of the nitrile more electrophilic. This facilitates the nucleophilic addition of alcohols, which leads to the formation of an imidic salt intermediate. From here, the reaction proceeds through a standard imine hydrolysis, similar to what we saw in the chapter on aldehydes and ketones. Water attacks the imine carbon, leading to its breakdown and forming the final ester product.
Let’s also mention a couple of interesting approaches for converting nitriles to esters.
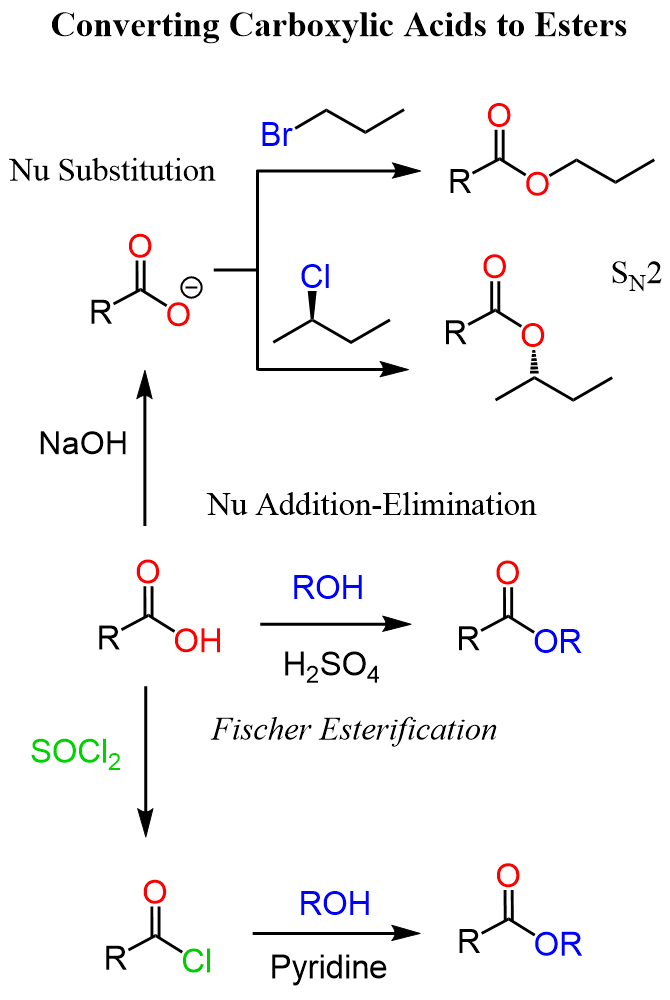
Nitriles to Esters via Carboxylic Acids
We know that nitriles can be hydrolyzed under acidic or basic conditions to carboxylic acids:

Once we have the carboxylic acid, we can either subject it to a Fischer esterification or convert it into a carboxylate salt and use it for an SN2 substitution with an appropriate alkyl halide.
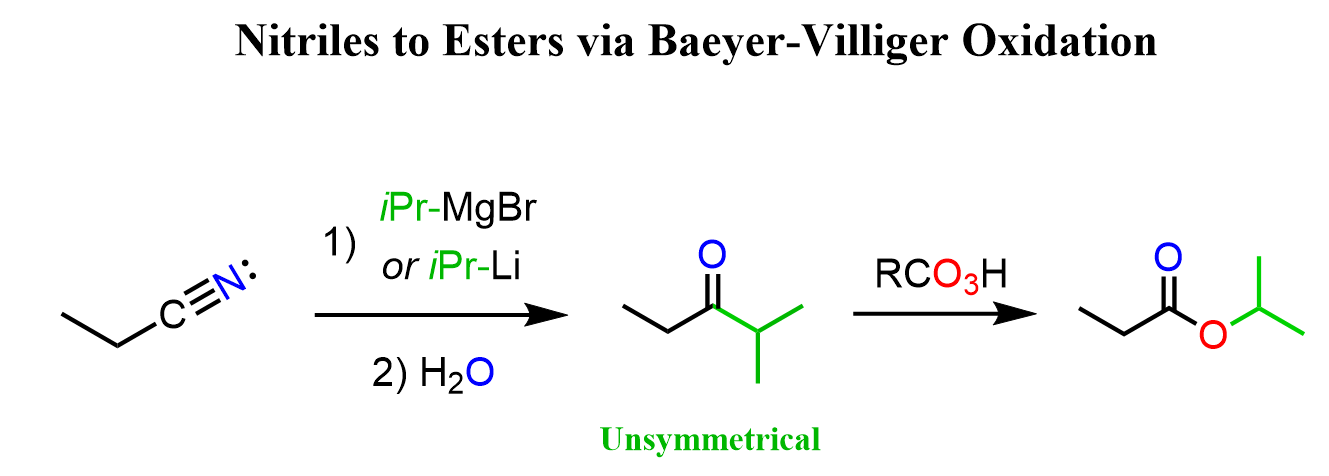
Nitriles to Esters with Ketones
You might remember that nitriles react with Grignard and organolithium reagents to form ketones. Once we have the ketone, we can use the Baeyer-Villiger oxidation to convert it into an ester:
Check the linked article for more details on the mechanisms of all the reactions mentioned in this post, which is dedicated to summarizing strategies for converting nitriles to esters.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- The reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz

