In this chapter on aldehydes and ketones, we are going to see a new type of reaction – the nucleophilic addition to the carbonyl (C=O) bond:

The nucleophilic addition to the carbonyl group occurs as a result of the imbalance in the electron density between the carbon and oxygen of the C=O bond. Oxygen is more electronegative, and therefore, the carbon is electron-deficient, and thus susceptible to a nucleophilic attack by a variety of electron-rich species:
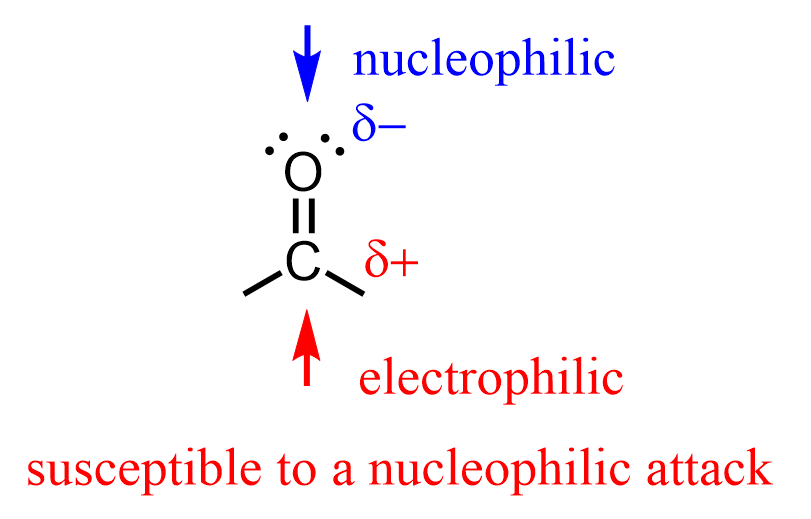
We can see this by also drawing the second resonance structure of the carbonyl group where the carbon is positively charged:
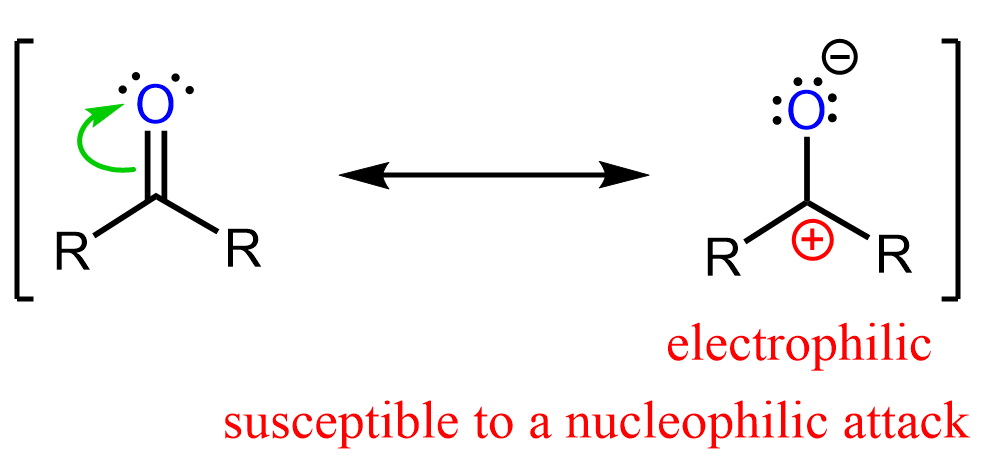
Notice that after the nucleophilic addition, the hybridization of the carbon changes from planar sp2 to tetrahedral sp3 hybridization.
The Reactivity of Aldehydes and Ketones
In general, aldehydes are more reactive to nucleophilic additions than ketones which (and this is largely true for any organic reaction) is explained by two factors: electronic and steric. The electronic factor is the electron distribution between two atoms, or we can say how uneven it is. The steric factor is how much the reactive center is hindered by other groups which make the reaction more difficult.
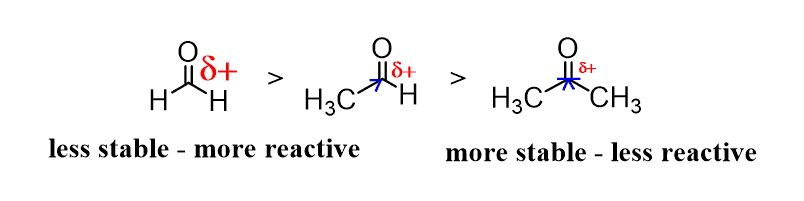
Let’s discuss the electronic effect for comparing the reactivity of aldehydes and ketones in nucleophilic addition reactions. The partial positive charge of the carbon in a ketones is suppressed by the two alkyl groups (remember, alkyl groups are electron donors) and therefore, aldehydes are more reactive than ketones:
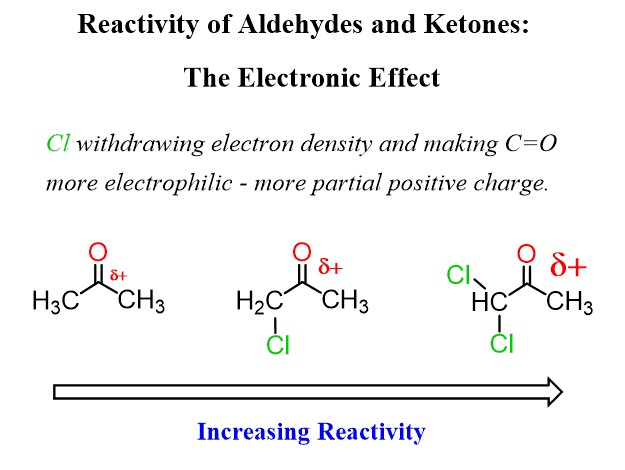
Consequently, electron-withdrawing groups near the carbonyl carbon decrease the its electron density, thus making it more reactive in nucleophilic addition reactions.
On a side note, remember that the electrophilicity of the carbon atom of the ester is partially suppressed by the lone pair of the oxygen through resonance stabilization and they are less reactive than ketones:

And now the steric effect. The tetrahedral product of the addition reaction is less crowded/bulky/sterically hindered and hence more stable in the case of aldehydes:

Also, (bulkier) alkyl groups of ketones make the nucleophilic attack more difficult/slower. The nucleophilic addition to carbonyl group occurs both in acidic and basic media although considering that many nucleophiles such Grignard, Gillman, organolithiums, amines etc., are bases, we can say that the addition under basic conditions is more common and important.
Nucleophilic Addition Under Acidic Conditions
Nucleophiles are Lewis bases as they are the electron donors, so acidic media should suppress their reactivity. The reason for acids catalyzing the nucleophilic addition is the protonation of the oxygen in the carbonyl group. Once protonated, the electron-withdrawing inductive power of the oxygen increases as it is now positively charged. In other words, protonation activates the carbonyl group for a nucleophilic addition:
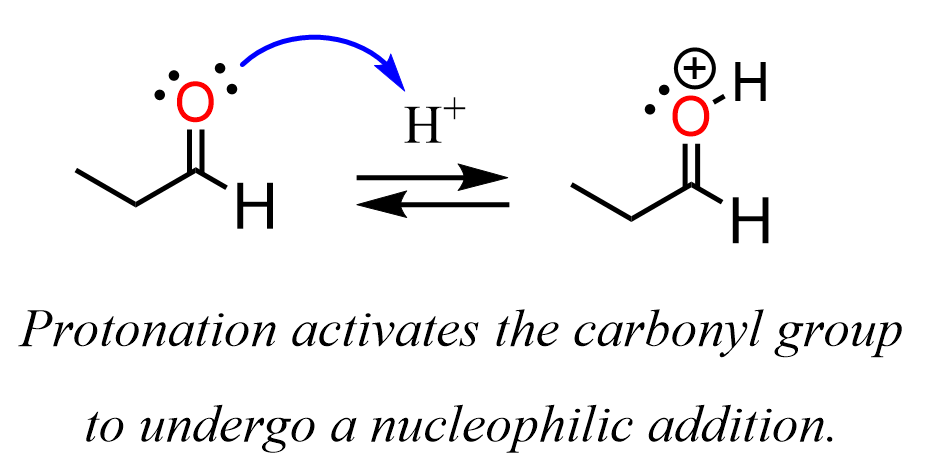
This is a pretty common theme, so keep this sequence in mind for the upcoming lots of these reactions.
An example of an acid-catalyzed nucleophilic addition to the carbonyl group is the hydration of aldehydes and ketones. Once, the protonation happens, the carbonyl is activated, and water now acts as a nucleophile attacking the carbonyl group:

Nucleophilic Addition Under Basic Conditions
Although very often we do not show the counterion of the nucleophile, it is still there, and plays the same role as the proton in activating the carbonyl group. This occurs via coordination to the oxygen which we can see for example, in the hydride reduction of carbonyl compounds by LiAlH4 and NaBH4:

The first part of this particular reaction is an example of a nucleophilic addition-elimination mechanism, which we will see more in the chapter on carboxylic acid derivatives, but I just wanted to give you an example of how counterions activate the carbonyl group.
An easier example can be the hydration of aldehydes and ketones under basic conditions. Here, the nucleophile is –OH, which is a stronger nucleophile than water and
attacks the carbonyl carbon, cleaving the π bond, and moving an electron pair onto oxygen. This forms a negatively charged intermediate which, upon protonation by water, results in a geminal diol:

As a short summary, we can put the following for the nucleophilic addition to the carbonyl group:

The nucleophilic additions of aldehydes and ketones can be seen in a variety of their reactions, such as with water, amines, cyanide, Grignard and etc. The links to these articles and practice problems are listed below.
- Nomenclature of Aldehydes and Ketones
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Reduction of Carbonyl Compounds by Hydride Ion
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- Grignard Reaction with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones

??????????????? why is this empty
Notes???
I am currently working on different content material, and it is taking all my time. Please check the other topics on the reactions of aldehydes, ketones such as the addition of water, alcohols, and amines – they all are examples of nucleophilic addition to the carbonyl group. I will write about the principle and general mechanism of these reactions once I get a chance.
Is there anything about this in the cheat sheet? I’m willing to buy it I just wantedto make sure the content I needed is in there first. I understand that it takes a while to make a great resource. Your hard work and passion is appreciated
Can you explain why anhydrides are more reactive than aledhyes but esters aren’t even though both have resonance please ?
Let’s first make sure we are on the same page when comparing the reactivity of aldehydes and ketones with esters. The latter are less reactive because of the resonance donation by the oxygen of the alkoxy group. This reduces the partial positive change on the carbonyl carbon thus makes it less reactive towards nucleophilic additions.
Now, the same can be said about anhydrides, however, if you draw a resonance structure of an anhydride, you will see that one of the carbonyl carbons appears next to a positively charged oxygen and therefore, it is more reactive than in aldehydes and especially in esters. The second factor is the stability of the leaving group. While in the case of an ester, the leaving group is alkoxide ion, the reaction of anhydrides expels a carboxylate ion which is resonance stabilized thus it is a better leaving group.
Thank you so much !!
You are welcome. Let me know if you have more questions.