Aldehydes and ketones react with alcohols under acidic conditions to form acetals. Acetals are tetrahedral compounds where two alkoxy (OR) groups are bonded to the central carbon atom.
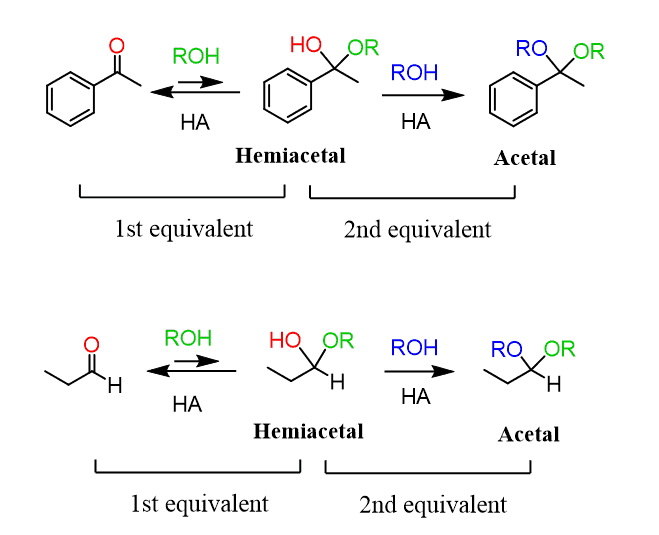
The mechanism of the reaction is similar to what we learned in the acid-catalyzed hydration of aldehydes and ketones. The are examples of nucleophilic addition-elimination reactions to the carbonyl group.
It starts with a protonation of the carbonyl oxygen, which makes the C=O carbon highly electrophilic:
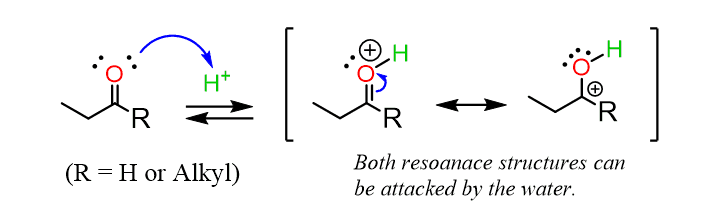
The carbon is then attacked by the alcohol, forming an oxonium intermediate, which is then deprotonated to form a hemiacetal. Notice that the hemiacetal has only one alkoxy group compared with the two acetals:

In the next step, the OH group of the hemiacetal is protonated and converted into a good leaving group:
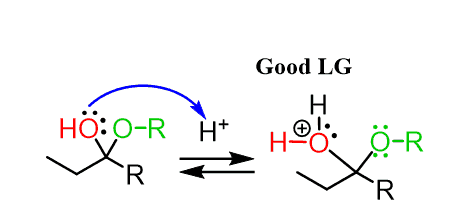
This may look ready to be attacked by another alcohol molecule, kicking out H2O.
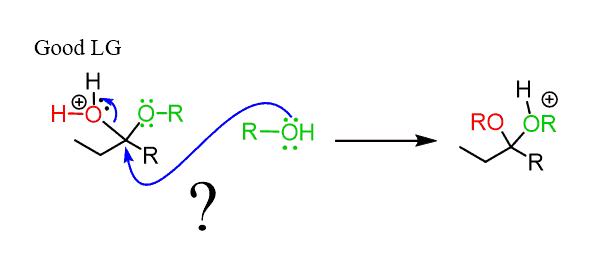
However, notice that the carbon is sterically hindered and therefore, no SN2 can occur here.
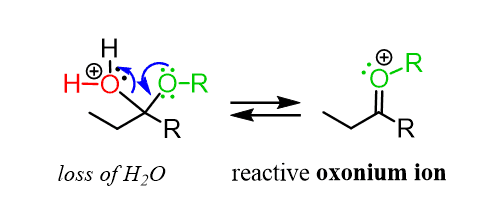
Instead, the reaction goes by an SN1 mechanism where the leaving group is first expelled by the lone pairs of the neighboring oxygen, which forms another oxonium intermediate readily available to react with an alcohol nucleophile:
After the nucleophilic attack, we have the deprotonation of the oxonium intermediate, generating an acetal:
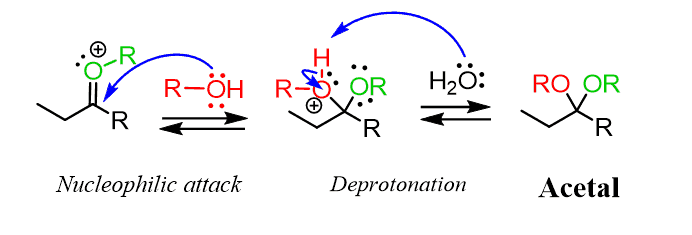
Let’s put this mechanism of acid-catalyzed formation of acetals in a nice summary:

How are Acetals Isolated if the Reaction is at Equilibrium?
Just like the hydration, the equilibrium favors reactants rather than products except for some simple or more reactive aldehydes. This has to do with the electronic and steric effects, and we covered it in the acid-catalyzed hydration of aldehydes and ketones.
There is, however, an important difference to consider when comparing the formation of hydrates and acetal!
Hydrates are prepared in aqueous solutions which means the water is in an enormous excess.
The synthesis of acetals, on the other hand, produces water as a by-product. This is a great advantage since the equilibrium can be shifted to the right by removing the water as it is formed and forcing the completion of the reaction:
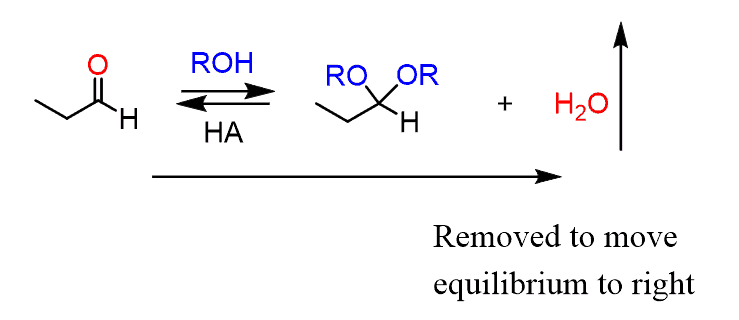
For this, either a drying agent or a special distillation setup (Dean-Stark trap) can be used.
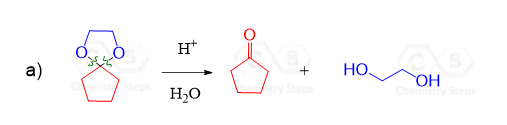
Cyclic Acetals
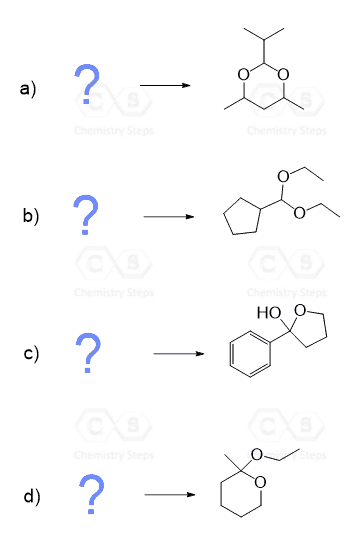
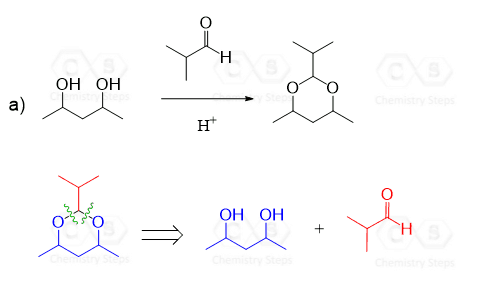
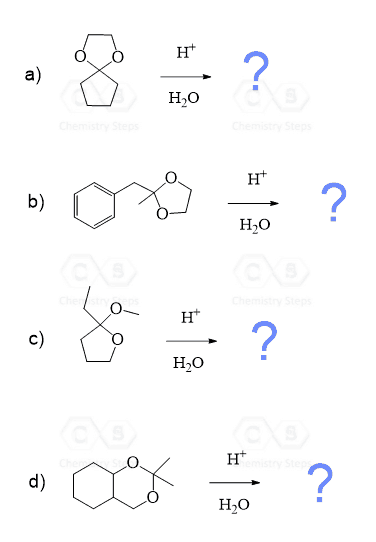
Notice again that acetals contain two alkoxy groups and therefore, two equivalents of alcohol are reacted with the aldehyde or ketone to produce them.
These two equivalents of the OH group can also be incorporated by a diol – an alcohol that contains two OH groups. This reaction produces a cyclic acetal, which is especially favored when five or six-membered rings are formed:

The mechanism of this reaction is the same as what we saw for the formation of regular acetals. Can you draw it? Simply use the OH of the diol instead of the second equivalent of the alcohol.
Cyclic acetals are very useful as protecting groups for aldehydes and ketones in basic conditions. More details will be covered in the next post.
Acetals and Carbohydrates
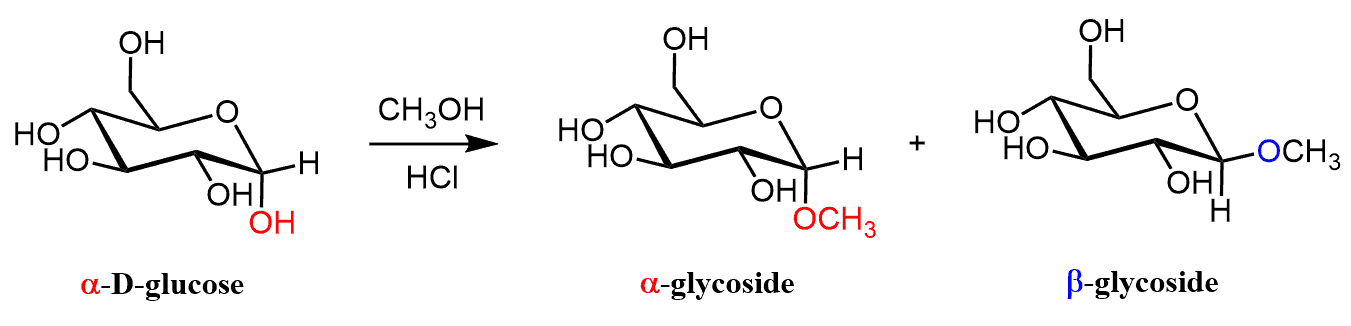
An important class of acetals is the glycosides. These are the acetals of monosaccharides, which are the simplest carbohydrate molecules.
For example, the treatment of glucose with methanol and HCl forms two isomeric glycosides because glucose also exists in an open-chain form with an aldehyde group: