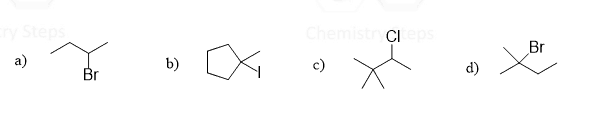
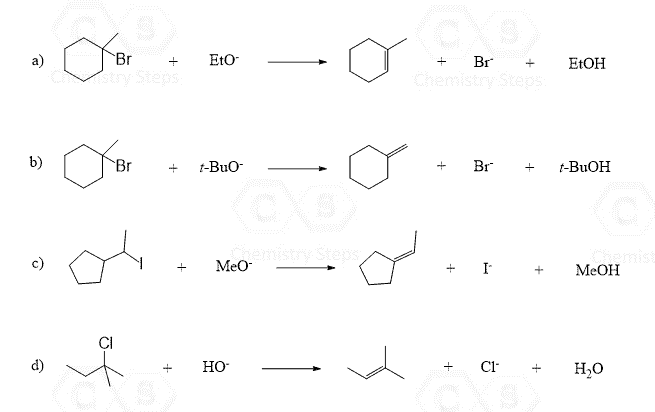
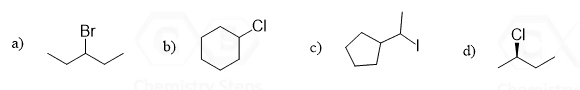Let’s make an overview of elimination reactions in organic chemistry. The first question we want to address is: What is eliminated from the molecule?
While there may be different atoms removed in elimination reactions, most of the time it involves the removal of a halogen or an OH group together with a neighboring hydrogen, which results in the formation of a double bond.
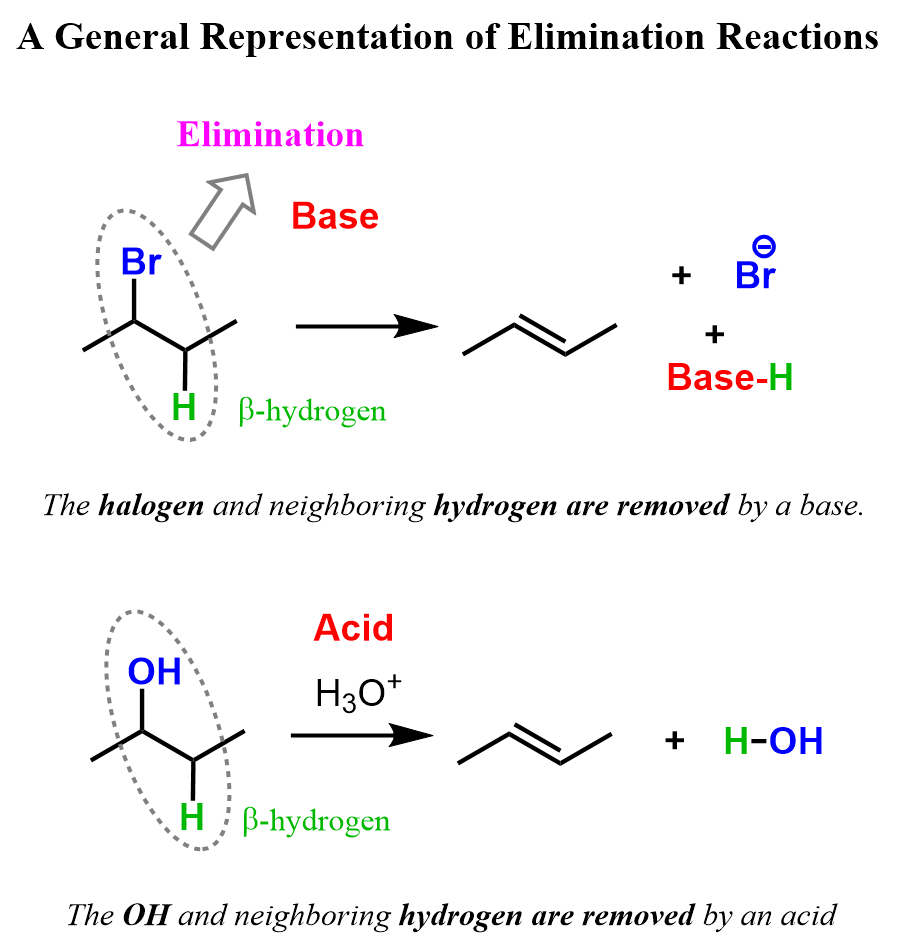
So, what do we need to remove the halogen or an OH group together with the neighboring hydrogen (β-hydrogen)? To answer this question, let’s mention first that there are two main mechanisms of elimination: E2 and E1.
The E2 Elimination Mechanism
In the E2 mechanism, a strong base attacks the β-hydrogen, breaking the C–H bond and pushing the electrons onto the carbon connected to the halogen. This shift of electrons forms a new π bond, simultaneously breaking the C–halogen bond.
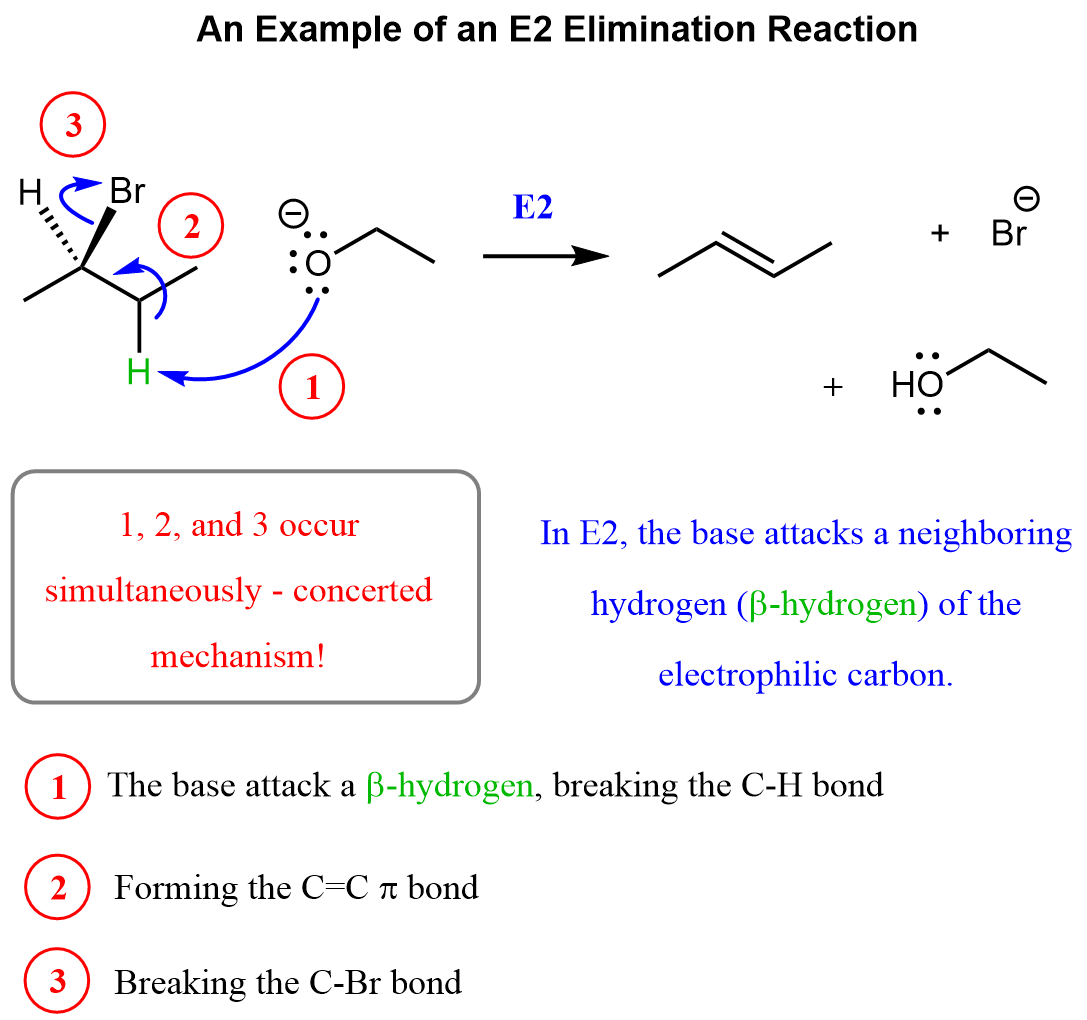
The reason this is called an E2 mechanism is that it is a bimolecular elimination – the rate of the reaction depends on both the substrate and the base. In other words, the base and the alkyl halide participate together in a single, concerted step to form the alkene.
Therefore, the numbering of arrows here is to simply label and explain them rather than show the sequence of steps – they all happen simultaneously.
Like in nucleophilic substitution reactions, the halogen here is called a leaving group, and we can have Cl, Br, or I. Aside from the halogens, mesylates and tosylates are the most common leaving groups in nucleophilic substitution and elimination reactions.
There are many strong bases you will see in your Organic Chemistry 1 class, and we will list the most common ones below:
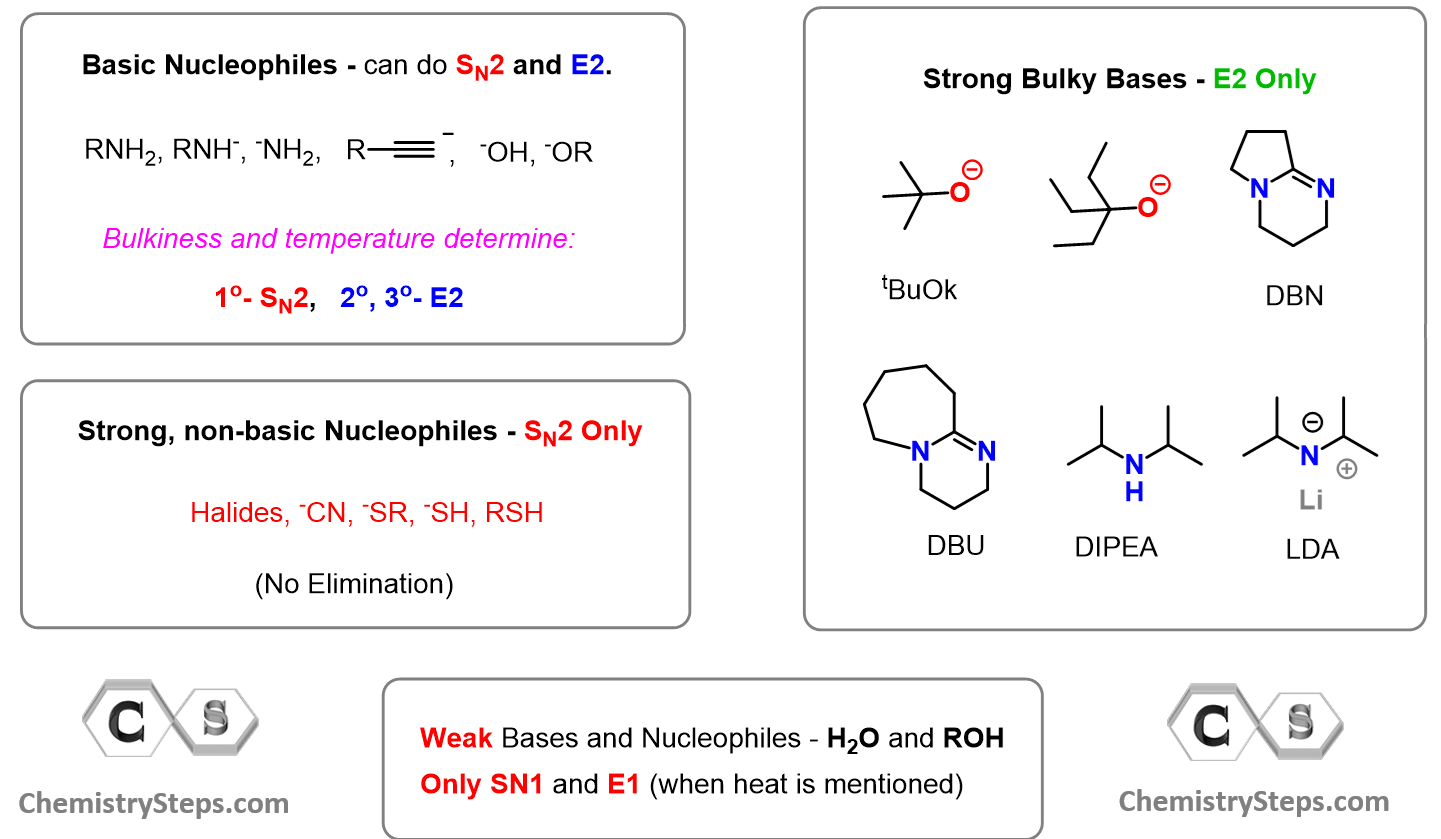
Notice that some of the bases can also act as good nucleophiles and participate in SN2 nucleophilic substitution reactions. Deciding which mechanism predominates is a different topic that we won’t discuss here, but you can read about it in this dedicated post.
The E1 Mechanism
The E1 mechanism occurs when a weak base, such as water or an alcohol, is used. The beta hydrogen is only removed after the loss of the leaving group, when a carbocation is formed.

So, one way you can remember this is that the weak base is reluctant to attack the beta hydrogen, and it only does so after a carbocation is formed. The carbocation is so unstable that even a “lazy” weak base attacks the beta hydrogen to break the C–halogen bond and provide electrons to the positively charged carbon.
Let’s put the E1 and E2 elimination reactions of alkyl halides together so we have a better visual comparison of them:

Elimination Reactions of Alcohols
Although halogens are still the most commonly encountered leaving groups in E1 elimination, you need to know that alcohols also form alkenes via this mechanism, like we showed in the first two reactions.

What happens here is the protonation of the OH group, converting it into a great leaving group, water:
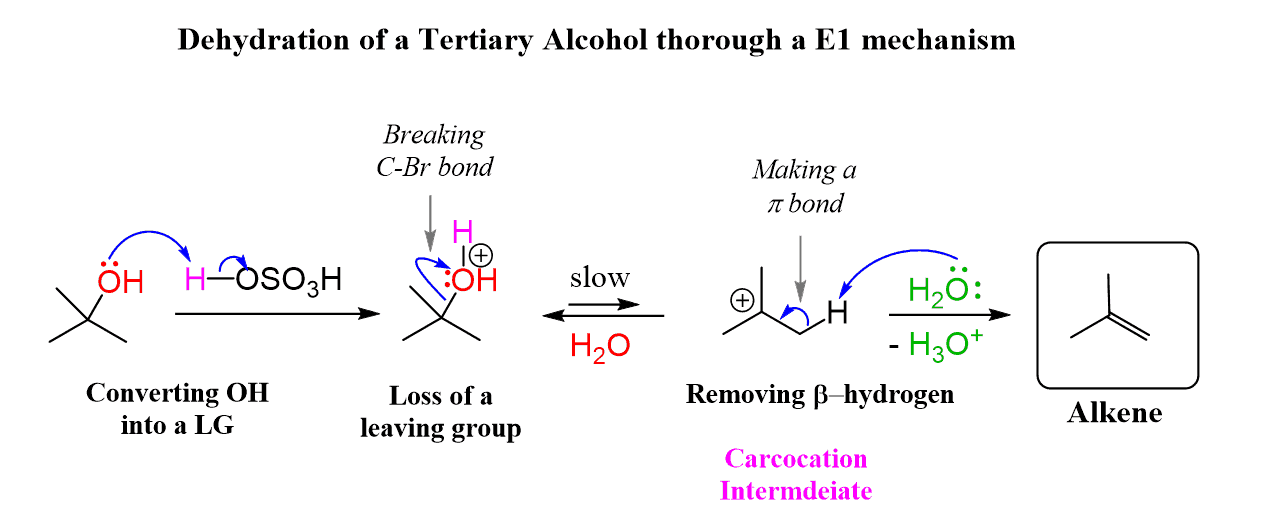
Remember that the hydroxide ion itself is not a leaving group because it is a strong base, thus very unstable and reactive.
One way of converting the OH into a good leaving group so that a strong base, and thus an E2 elimination, can be used is their conversion to mesylates and tosylates. A mesylate or tosylate is equivalent to an alkyl halide; therefore, they undergo all the substitution and elimination reactions that alkyl halides do:
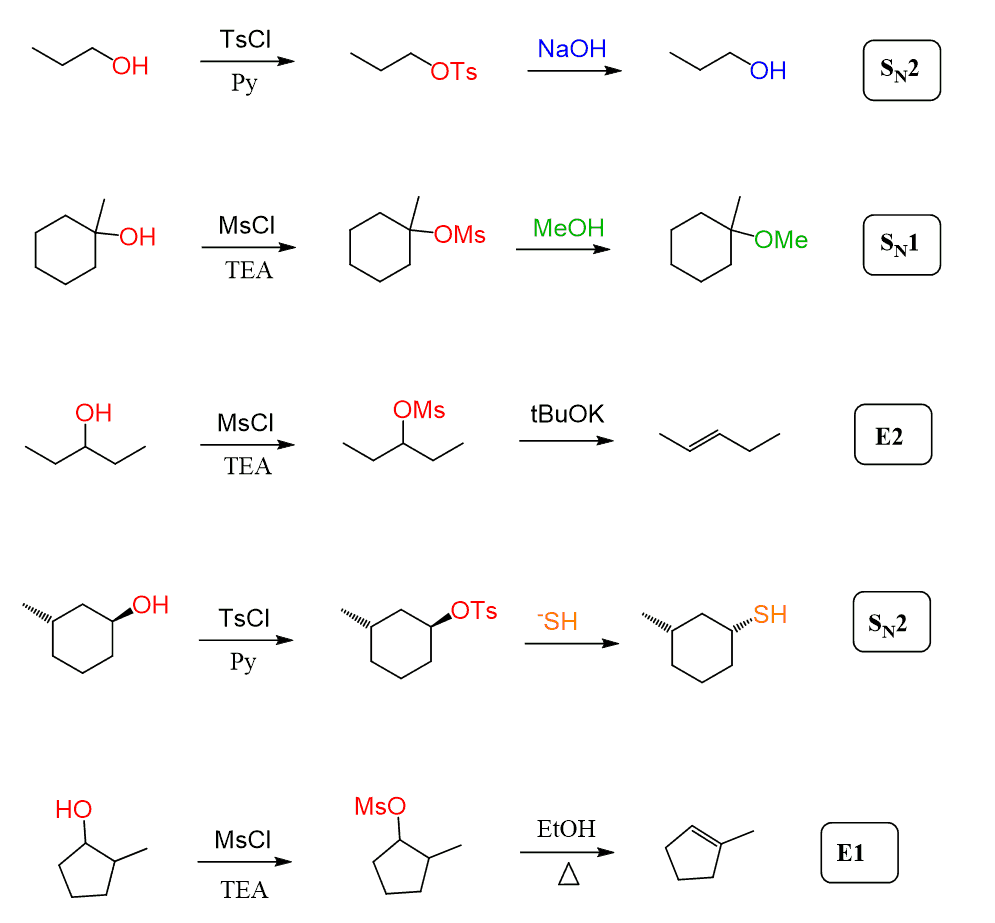
Check this article on mesylate sand tosylates for more details.
The Regioselectivity of Elimination Reactions
Remember, the regioselectivity or regiochemistry of a reaction refers to which position in the molecule the new bond (or double bond, in this case) forms.
In elimination reactions, the general tendency is that the more substituted alkene is formed as the major product.
For example, if you treat 2-bromobutane with sodium ethoxide, but-2-ene is the major product, while but-1-ene is the minor product. When a sterically hindered base such as potassium tert-butoxide (tBuOK) is used, the less substituted alkene, known as the Hofmann product, is formed:

This is because the more substituted alkene is more stable, due to hyperconjugation and inductive effects from neighboring alkyl groups that help delocalize electron density around the double bond.

The same tendency is observed for the E1 mechanism – wherever possible, the more substituted alkene is formed.

One thing to be cautious of in E1 reactions is the possibility of rearrangements, because they proceed via the formation of a carbocation. Remember, carbocation rearrangements occur when a more stable carbocation can form via a hydride or methyl shift.
For example, when 2-chloro-3,4-dimethylpentane is heated in a solution of ethanol, 2,3-dimethylpent-2-ene is formed instead of the expected 3,4-dimethylpent-2-ene because of a 1,2-hydride shift that transforms the secondary carbocation intermediate into a more stable tertiary carbocation.
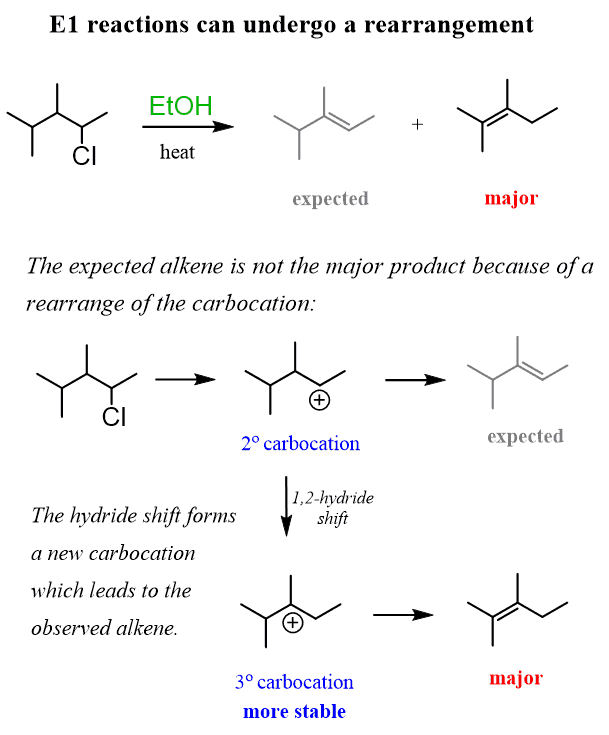
There are different ways of addressing the rearrangements in E1 elimination reactions, but we cannot cover them all in this overview post. Check out the linked articles in the text and after the post for more information.
Summary of Elimination Reactions
In elimination reactions, atoms or groups are removed from adjacent carbon atoms, typically a hydrogen (β-hydrogen) and a leaving group such as a halogen or an OH group. The result is the formation of a double bond – an alkene.
There are two main types of elimination mechanisms: E2 and E1.
- The E2 mechanism is a one-step, concerted process that requires a strong base. The base removes a β-hydrogen while the leaving group departs simultaneously, forming the π bond.
- The E1 mechanism occurs in two steps: first, the leaving group departs to form a carbocation, and then a weak base removes a β-hydrogen to generate the alkene.
Alcohols can also undergo elimination, typically via an acid-catalyzed E1 mechanism after the hydroxyl group is protonated to form water, a much better leaving group. Alternatively, the OH group can be converted into mesylates or tosylates, allowing E2 elimination with strong bases.
In terms of regioselectivity, elimination reactions usually follow Zaitsev’s rule, forming the more substituted (and more stable) alkene as the major product. However, when bulky bases are used, the Hofmann product (less substituted alkene) can dominate.
Rearrangements are possible in E1 reactions because they occur via the formation of carbocation intermediates.
Check Also
- Substitution and Elimination Reactions
- The E2 Mechanism
- E2 Elimination Practice Problems
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- SN2 and E2 Rates of Cyclohexanes
- Elimination Reactions of Cyclohexanes with Practice Problems
- POCl3 for Dehydration of Alcohols
- The E1 Mechanism with Practice Problems
- Regioselectivity of E1 Reactions
- Stereoselectivity of E1 Reactions
- How to tell if it is E2 or E1 Mechanism
- SN1 vs E1 Reactions
- SN2 vs E2 Reactions
- Dehydration of Alcohols by E1 and E2 Elimination
- Mesylates and Tosylates as Good Leaving Groups
- Mitsunobu Reaction
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Polar Protic and Polar Aprotic Solvents
- SN1 SN2 E1 or E2 – the Largest Collection of Practice Problems
- The Hammond Postulate
- The E1cB Elimination Mechanism
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides