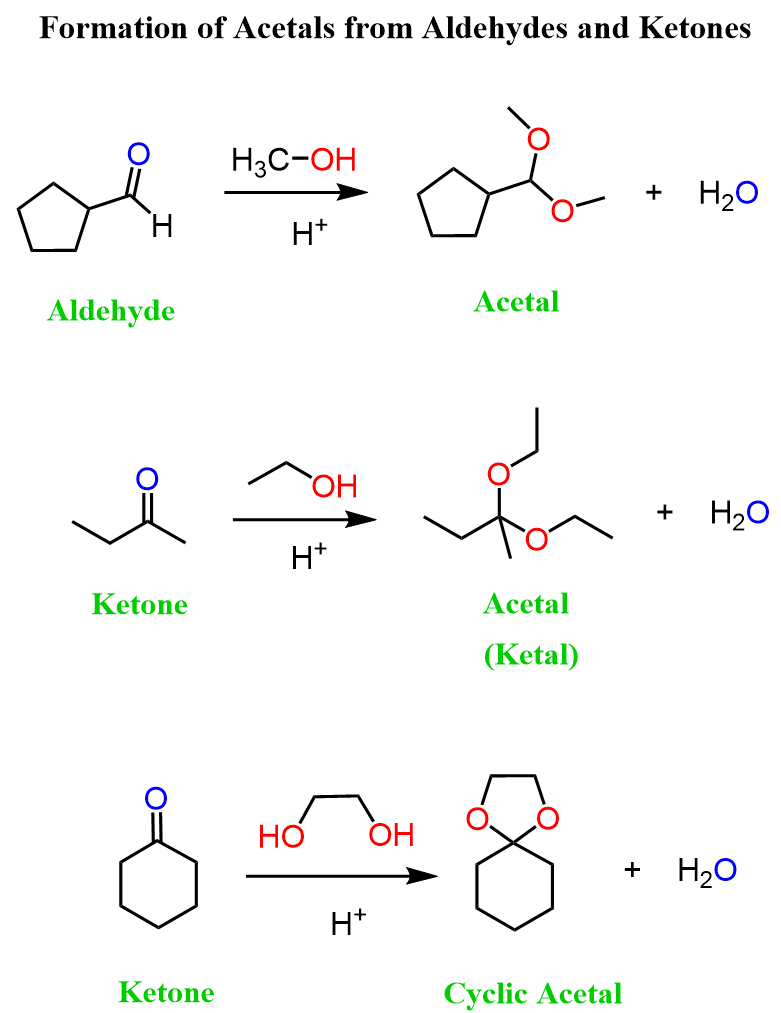
We have learned that the reactions of aldehydes and ketones with alcohols and amines result in the formation of acetals, imines, and enamines, depending on the structure of the amine:
These reactions are reversible, and one application of this feature was the use of acetals as protecting groups for aldehydes and ketones:

The acetal group protects aldehydes and ketones in basic conditions during, for example, LiAlH4 or NaBH4 reduction or Grignard reactions, after which it is removed by hydrolysis.
Remember, the hydrolysis is in equilibrium with the alcohol reaction, and to move the process forward, a large excess of water is used.
The question is the mechanism of the hydrolysis of acetals, and that’s what we will be discussing in today’s post.
Acetal Hydrolysis Mechanism
The reaction starts by protonation of one of the oxygen atoms, converting the alkoxy group into a good leaving group, which is then kicked out by the other oxygen:

The resulting oxonium ion is very electrophilic and is attacked by water, forming a hemicacetal after a deprotonation:

In a similar manner, the second alkoxy group is protonated and expelled by the hydroxyl group, forming a new oxonium ion, which, this time, is a protonated ketone:

And in the last step, the final product, ketone, is formed by another proton transfer reaction:

Putting all this together gives us a summary for the acetal hydrolysis mechanism:

Is there an Easier Way To Predict the Products of Hydrolysis?
There are quite a few steps in this reaction, but fortunately, you don’t need to remember all the steps in order to predict the structure of the aldehyde and ketone of an acetal hydrolysis.
Here is the shortcut: draw a line between each oxygen and the carbon to which they are connected.
These are the bonds that are cleaved during the hydrolysis. Each oxygen is part of the alcohol, and the carbon connected to them is the C=O carbon atom:

Additional examples of acetal, as well as imine and enamine hydrolysis, are covered in this practice problem set:
Check Also
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- Grignard Reaction with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones