Acetoacetic Ester Enolates Practice Problems
Practice
Determine the major product for each acetoacetic ester synthesis shown below:
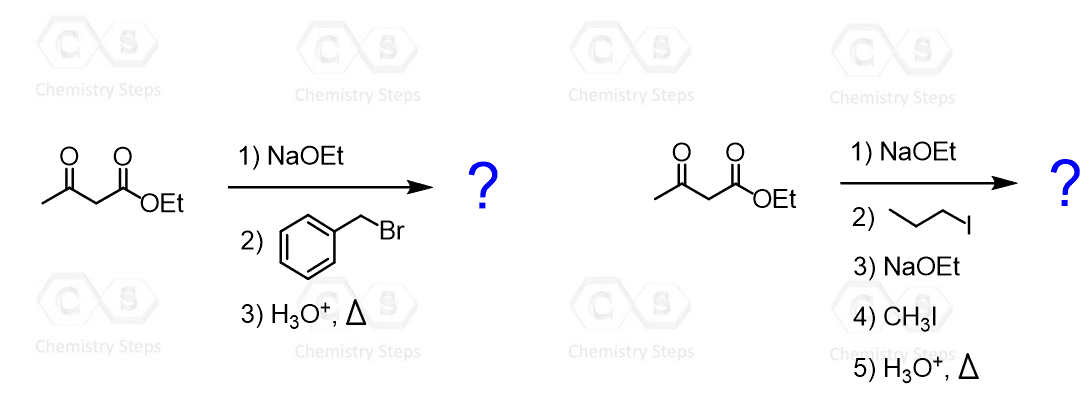
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
How would you use the acetoacetic ester synthesis to prepare each of the following products?
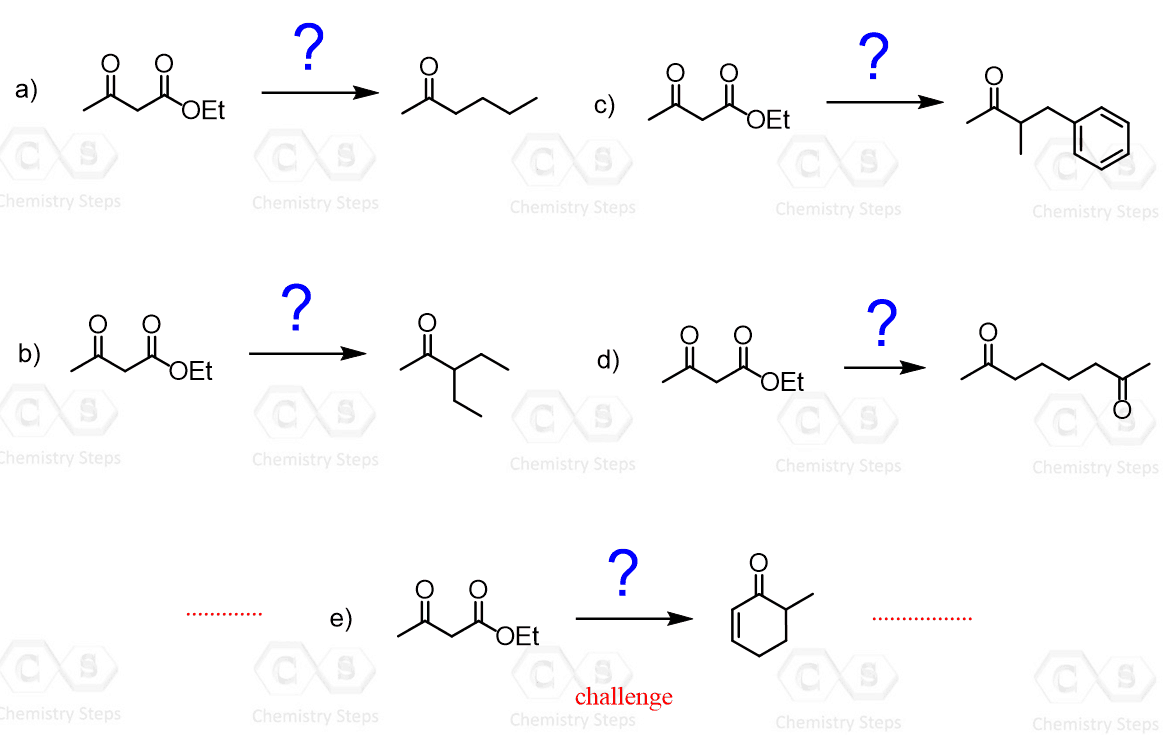
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem

hello Professor: I would like your opinion as to an alternate synthesis I have for your challenge problem above. I started as you did by producing the enolate ion and then adding a methyl group. From there I added 3-bromo-1-propanol, oxidized the OH group to an aldehyde, and then ran an Aldol using ethoxide using the methyl group on the keto portion of the AAE (acetoacetic ester). I hydrolyzed the ester, and then heated removing the carboxylate group and dehydrating the beta-hydroxy ketone I formed yielding the product.
I would like to know if my concepts are clear even if there is a mistake somewhere in the synthetic route.
Thanks for your time. A great web-site!
Hello,
Sounds like a feasible plan where only the decarboxylation step is done later. You can go ahead and email me (gevorg at chemistrysteps.com) your synthesis scheme so we can double-check.