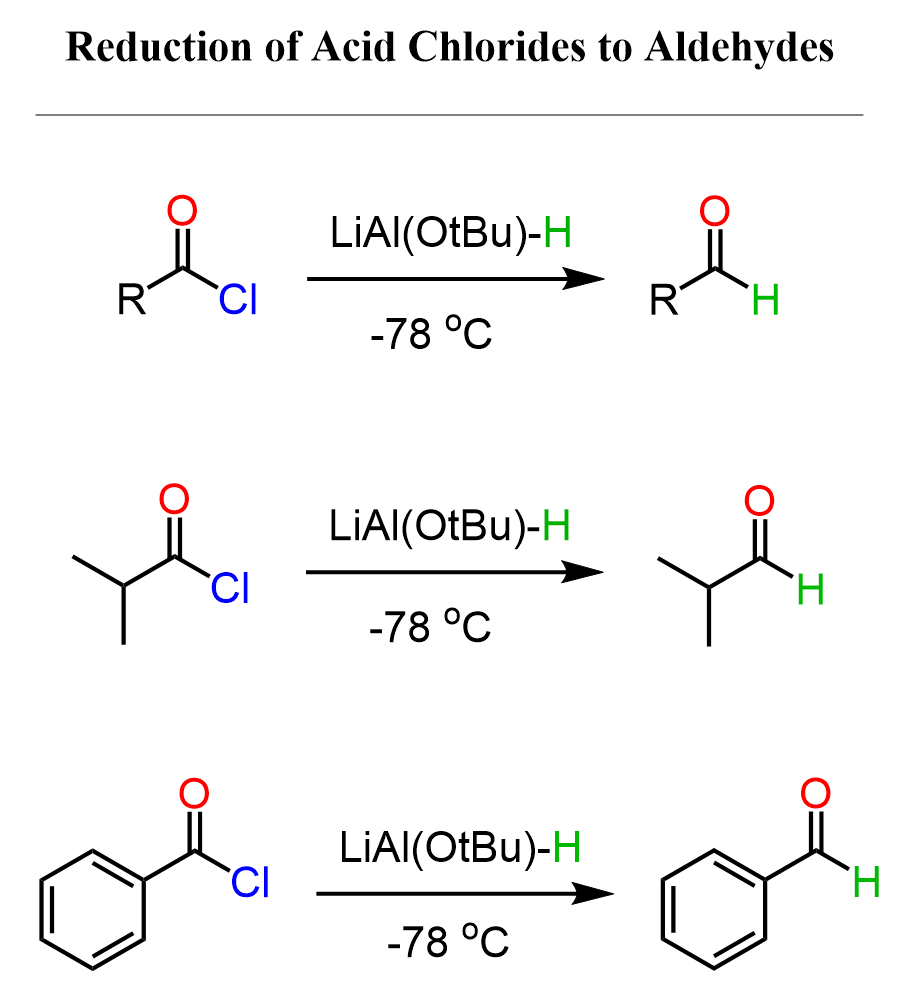
Acid chlorides can be reduced to aldehydes by lithium tri(t-butoxy) aluminum hydride (LiAl(OtBu)₃H):
So, why not use NaBH₄ or LiAlH₄ for reducing acid chlorides to aldehydes? Remember that acid chlorides are the most reactive derivatives of carboxylic acids, which makes it very difficult to stop their reactions with strong nucleophiles such as the hydride ion and organometallics. As expected, both LiAlH₄ and NaBH₄ reduce acid chlorides all the way to primary alcohols:
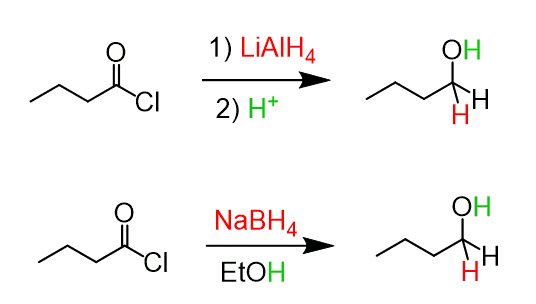
The reason for this is that the intermediate in these reactions is an aldehyde, which is still reactive towards NaBH₄ and LiAlH₄, and therefore, a primary alcohol is formed as the final product.
So, what makes LiAl(OtBu)₃H different than other reducing agents, specifically in the reduction of acid chlorides?
Let’s take a look at the structure of LiAl(OtBu)₃H:
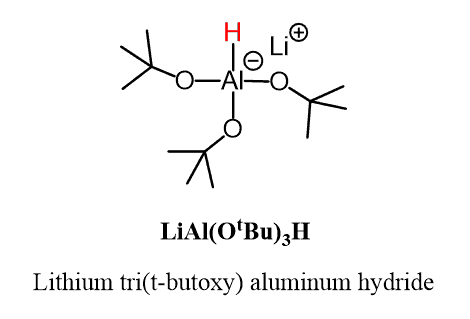
If we replace the three tBuO (tert-butoxide) groups with hydrogen, we’ll get LiAlH₄. So, apparently, these bulky groups make it less reactive than LiAlH₄, which allows us to stop the reaction at the formation of the aldehyde, especially if it is carried out at -78 °C.
The Mechanism of Acid Chloride Reduction
The mechanism of acid chloride reduction is similar to what we saw with LiAlH₄ and NaBH₄. It starts with a nucleophilic attack. The hydride ions from the reagent attack the carbonyl carbon of the acid chloride, pushing the π electrons up to the oxygen and forming a tetrahedral intermediate. Then, the intermediate collapses back down, and the chloride ion gets kicked out as a leaving group, giving us the aldehyde.

Once again, the bulky t-butoxy groups make the hydride source less reactive, so the aldehyde is not reduced further to a primary alcohol.
An alternative method for converting acid chlorides to aldehydes is catalytic hydrogenation using palladium and a poisoned (weakened) catalyst such as BaCO₃ or BaSO4, similar to what we saw with Lindlar’s reagent for the partial hydrogenation of alkynes to cis alkenes. This reaction is known as the Rosenmund reduction.
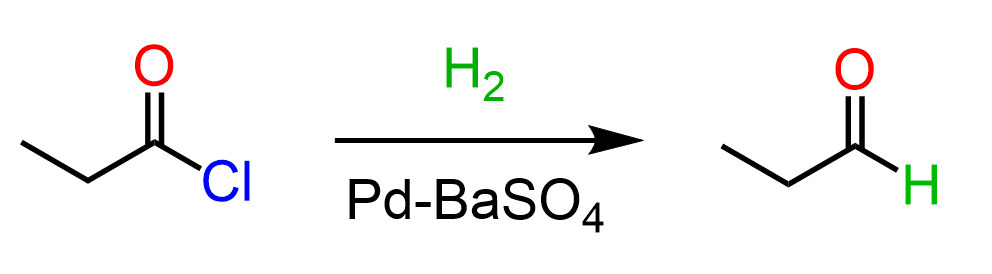
However, it’s less commonly covered in undergraduate textbooks, so chances are it might not come up in your class.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz

