Like any other hydrocarbon, alkynes are much less acidic than, for example, alcohols, thiols, carboxylic acids, and especially hydrohalic acids. However, what sets them apart from other hydrocarbons, such as alkanes and alkenes, is their relatively high acidity.
|
Compound Type |
Approximate pKa |
Example |
|
Alkane (sp³ C–H) |
~50 |
Ethane |
|
Alkene (sp² C–H) |
~44 |
Ethylene |
|
Alkyne (sp C–H) |
~25 |
Ethyne (acetylene) |
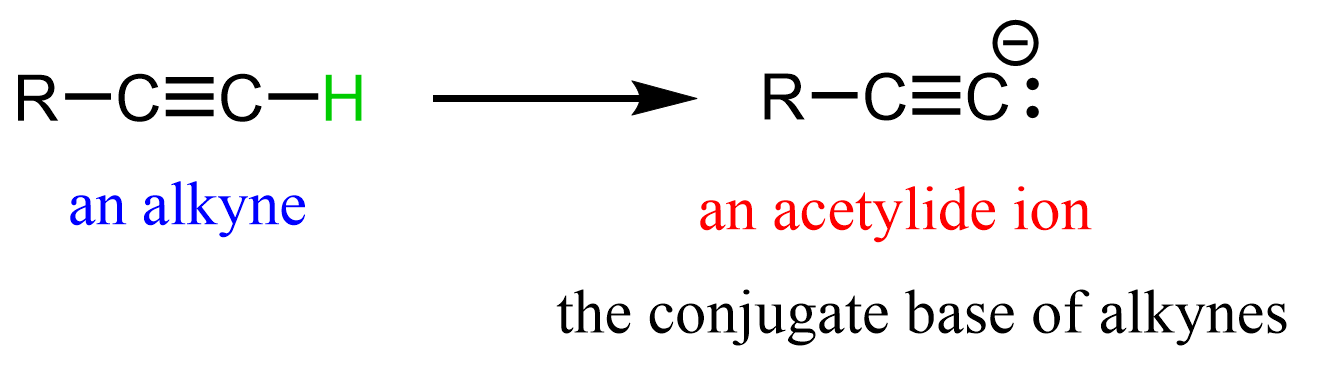
Of course, we are talking about terminal alkynes (general formula RC≡CH), as these are the only alkynes that have a hydrogen on the triple bond.
A pKa difference of 20 units means that the alkyne hydrogen is about 10²⁰ times more acidic than that of an alkane – a huge difference!
So, what makes the hydrogen connected to a triple-bonded carbon so much more acidic? We have the hybridization of the carbon atoms mentioned in the list above, and as you may have guessed, it has a significant effect here.
Remember, when discussing pKa and the factors that affect it, the key principle we learned was that the acidity of a molecule increases with the stability of its conjugate base. For alkynes in particular, this means the negatively charged carbon is much more stable when it is sp-hybridized rather than sp² and especially sp³.
This observation can be explained by the greater electronegativity of s orbitals compared to p orbitals. In an sp-hybridized carbon, the s-character is 50% (one s orbital and one p orbital). In sp² carbons, the s-character is 33%, and in sp³, it’s only 25%.

The greater the s-character of the orbital, the more electronegative it is, and thus, the better it stabilizes the negative charge.
The Application of Greater Acidity of Alkynes
The acidity of alkynes makes them unique compared to alkanes and alkenes because it allows them to be deprotonated under relatively mild conditions, most often using sodium amide:
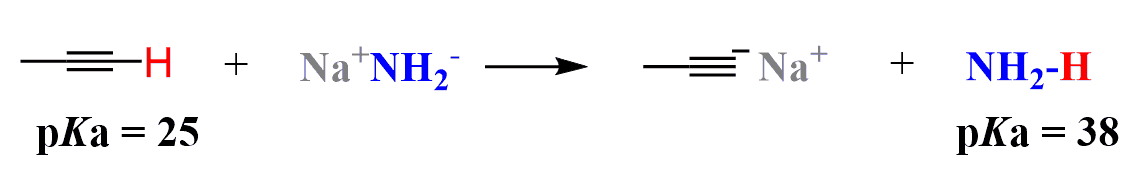
The resulting carbanion is called an acetylide ion, and it is an excellent nucleophile that can participate in a variety of SN2 substitution reactions.
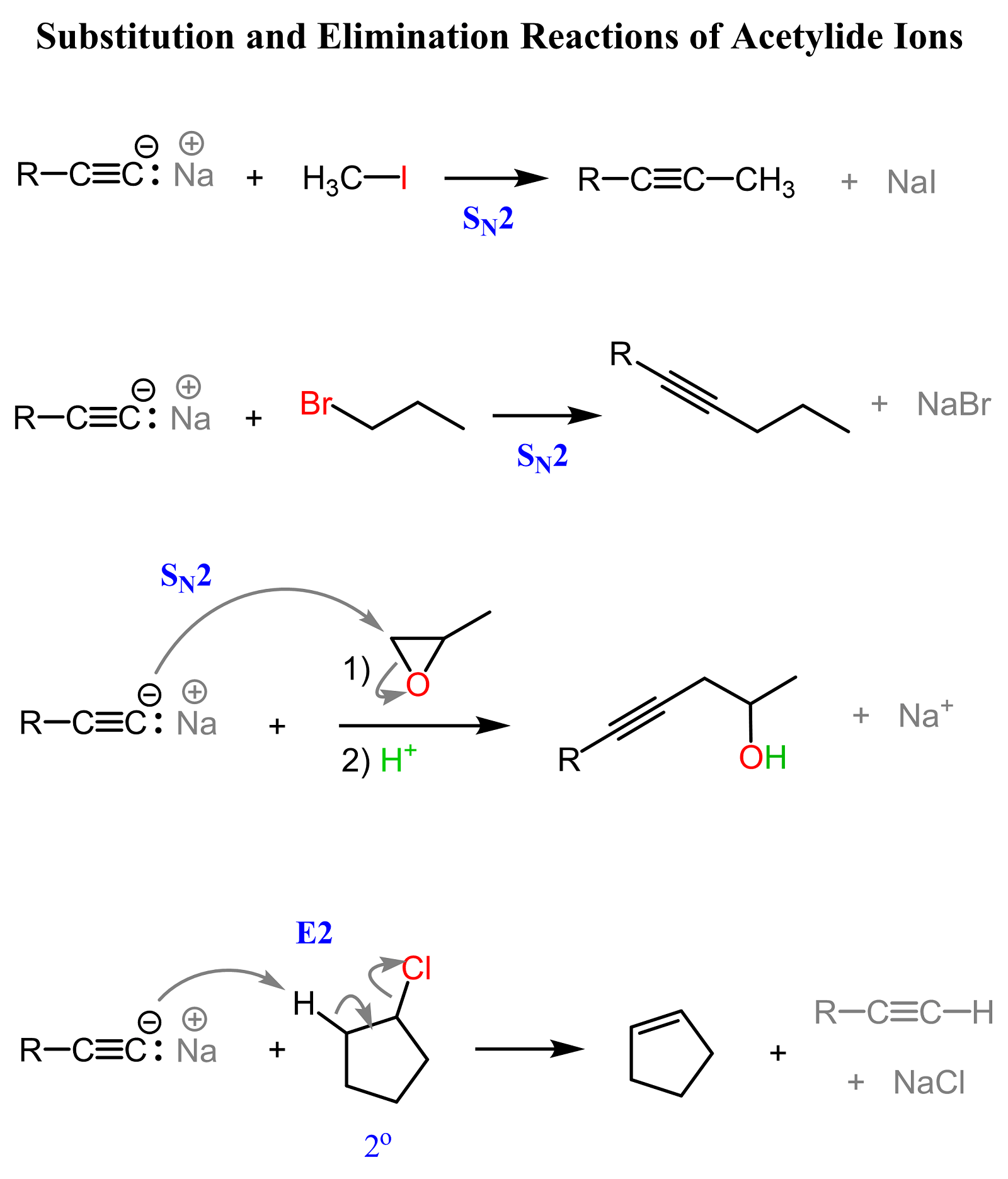
We have a separate post on the reactions of acetylide ions, so check it out for more examples and practice problems on how this concept is used in organic synthesis.
Check Also
- Introduction to Alkynes
- Naming Alkynes by IUPAC Nomenclature Rules – Practice Problems
- Preparation of Alkynes by Elimination Reactions
- Hydrohalogenation of Alkynes
- Addition of Water to Alkynes
- Acid-Catalyzed Hydration of Alkynes with Practice Problems
- Reduction of Alkynes
- Halogenation of Alkynes
- Hydroboration-Oxidation of Alkynes with Practice Problems
- Ozonolysis of Alkynes with Practice Problems
- Alkylation of Terminal Alkynes in Organic Synthesis with Practice Problems
- Reactions of Acetylide Ions
- Alkyne reactions summary practice problems
- Alkyne Synthesis Reactions Practice Problems
- Alkyne Naming and Reactions Practice Quiz
- Reactions Map of Alkynes
