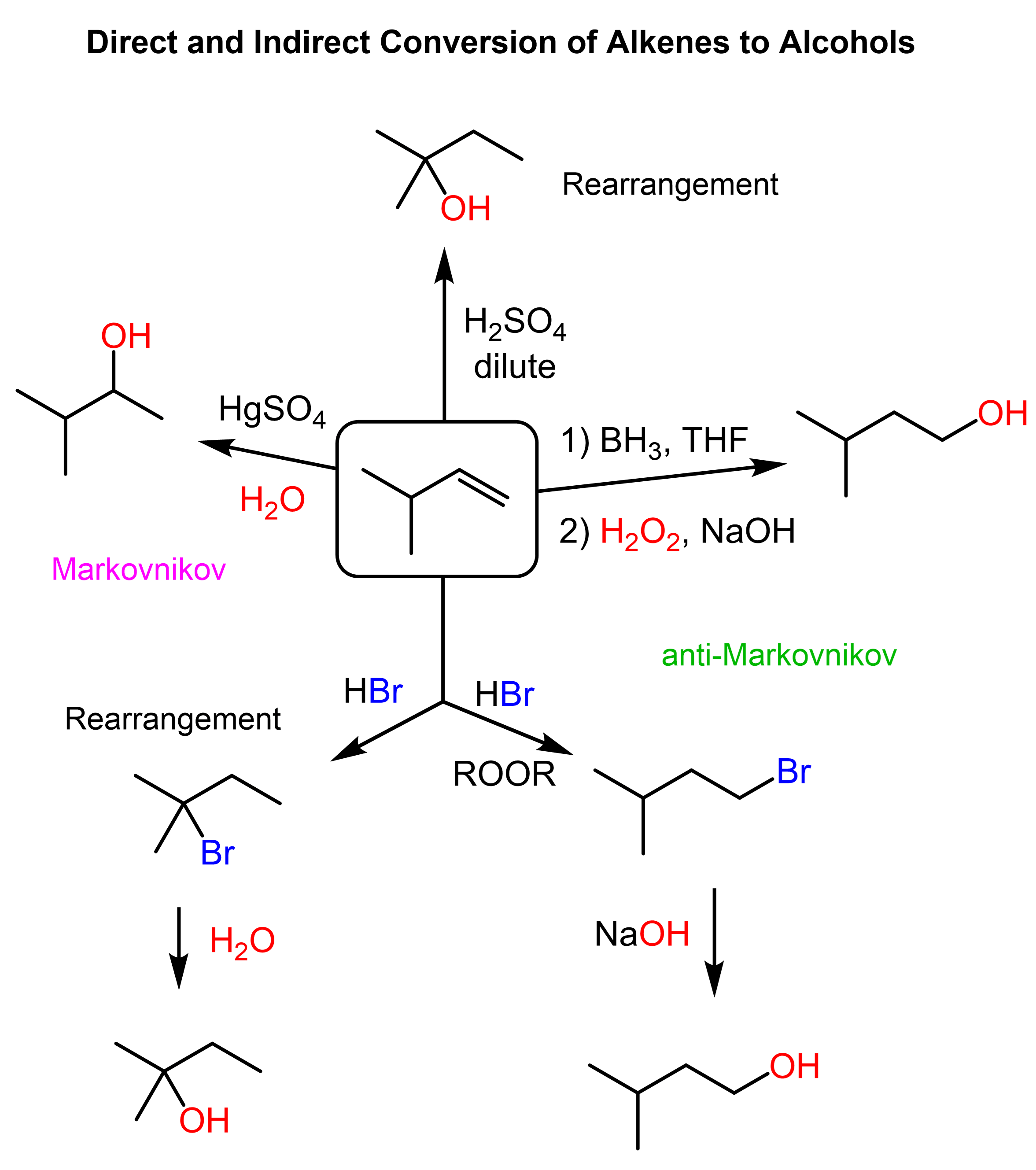
In the previous post, we discussed the methods for converting alkenes to alcohols via Markovnikov and anti-Markovnikov addition of water, the strategies to avoid possible rearrangements when the reactions go through carbocation rearrangements, as well as the indirect addition of the OH group via hydrohalogenations and substitution reactions.
Today, we’ll summarize the strategies for converting alcohols to alkenes. There are three main approaches we will discuss in this article: 1) Acid-catalyzed dehydration of alcohols, 2) Using POCl3 as a dehydration agent, and 3) Conversion via alkyl halides followed Zaitsev and Hofmann elimination.
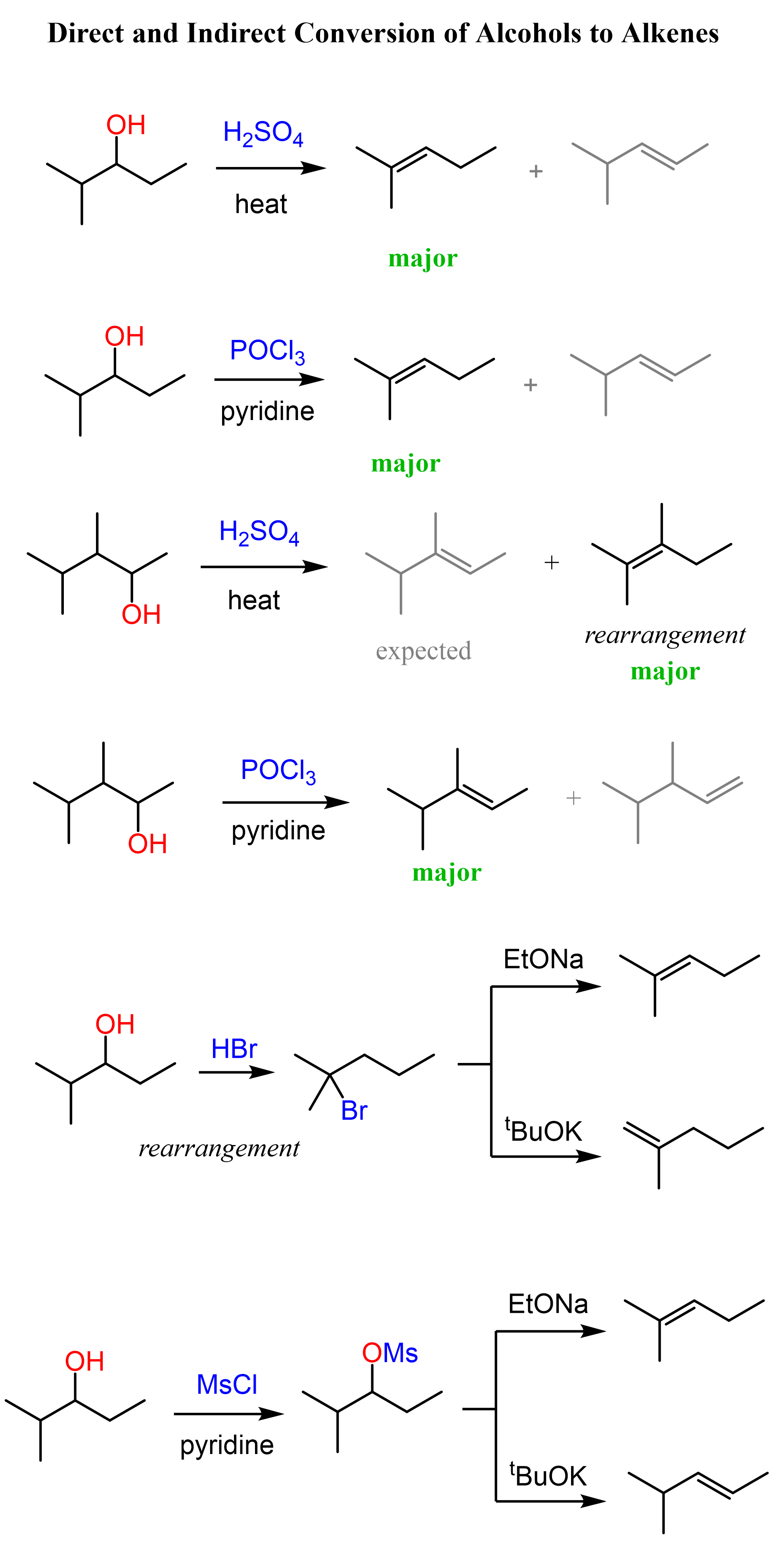
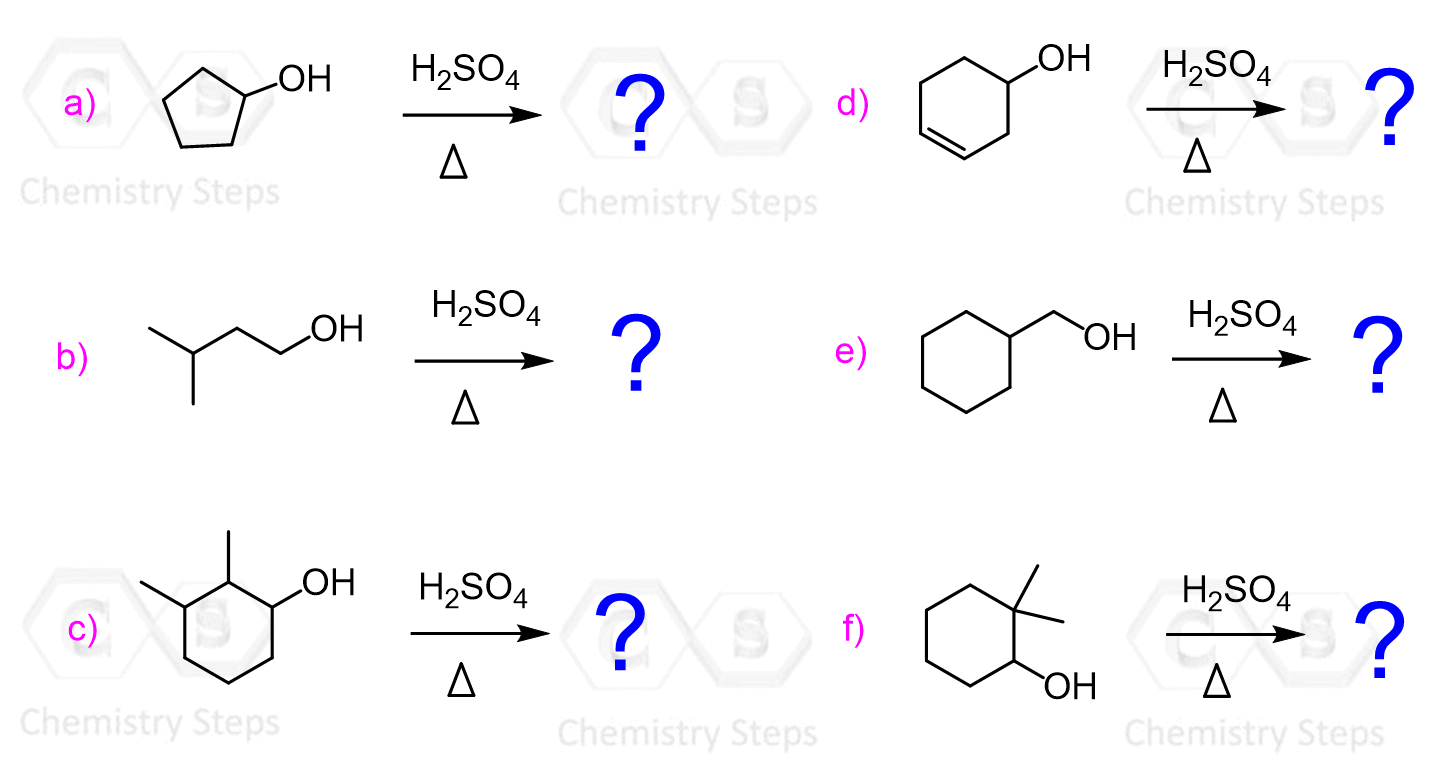
Let’s start with the dehydration of alcohols using strong acids such as H2SO4 and TsOH (p-Toluenesulfonic).
Acid-Catalyzed Dehydration of Alcohols
This method works for converting primary, secondary, and tertiary alcohols to alkenes.

Although for all the alcohols, the reaction starts with the protonation of the OH group converting it into a good leaving group, secondary and tertiary alcohols are dehydrated by E1, while primary alcohols by E2 mechanism.
You can check this article for more details about the mechanism of alcohol dehydration under acidic conditions.
Rearrangements in Dehydration of Alcohols
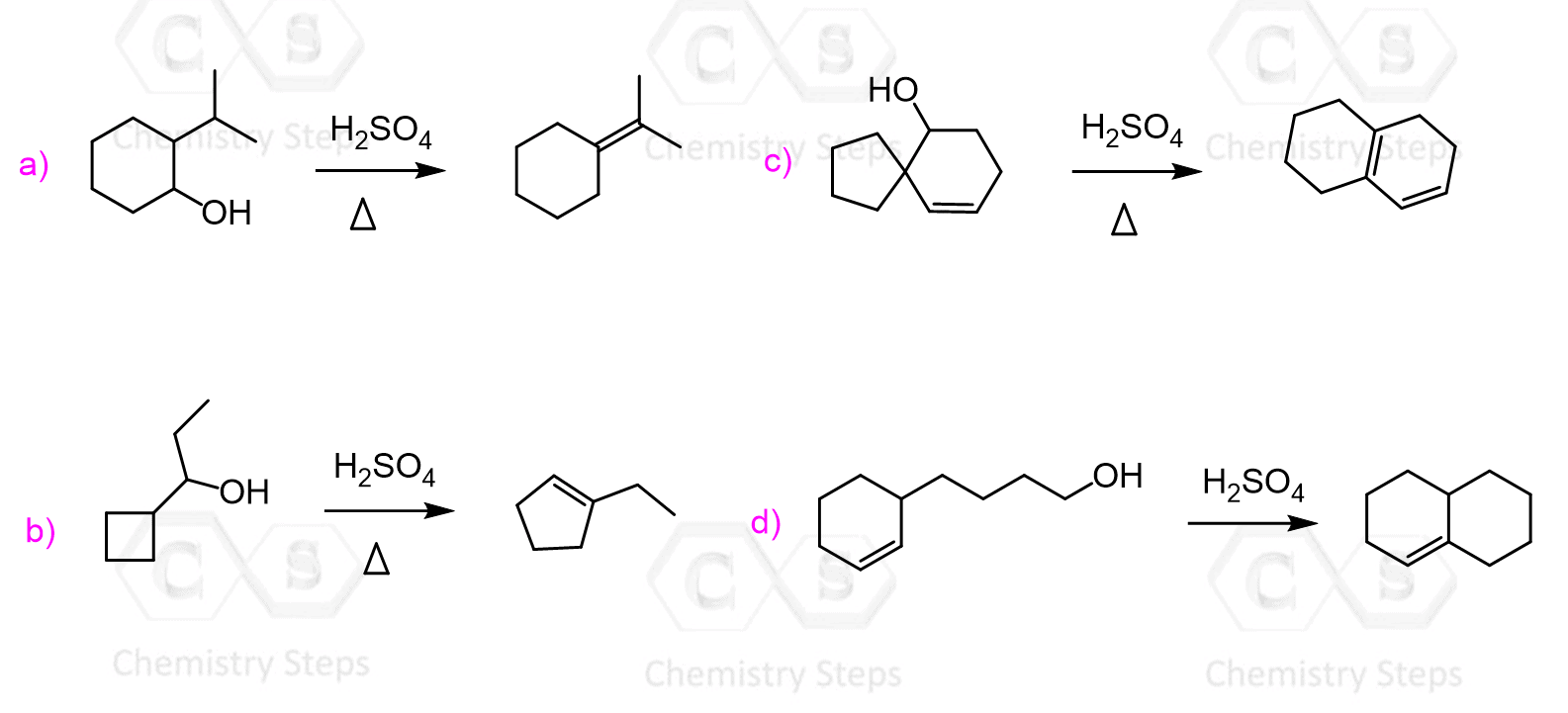
Possible rearrangements, alongside the harsh conditions associated with the use of concentrated sulfuric acid are the disadvantages of the dehydration of alcohols under acidic conditions. An example of a rearrangement is the dehydration of the following secondary resulting in a trisubstituted alkene as the major product:

We mentioned that primary alcohols do not form primary carbocations because they are too unstable, and a more stable carbocation is formed when a rearrangement is possible:

Alcohols to Alkenes using POCl3
Like the dehydration of the alcohols under acidic conditions, POCl3 also performs regioselective dehydration, and the more substituted alkene is formed.
The advantage of POCl3 is that no rearrangements are observed. For example, the rearrangement during dehydration of the alkene shown above can be avoided when POCl3 is used, and the alkene is formed according to the Zaitsev’s rule:

Let’s also compare the mechanisms of acid-catalyzed dehydration and the elimination using POCl3. In both reactions, the OH needs to be converted into a good leaving group, and POCl3 helps in this by converting it into –OPOCl2, much like the strong acid does in acid-catalyzed dehydration:

Once the hydroxyl is converted into a good leaving group, pyridine removes a β-proton which provides the electrons for making the C=C π bond.
The POCl3 elimination works for primary, secondary, and tertiary alcohols.
Indirect Conversion of Alcohols to Alkenes
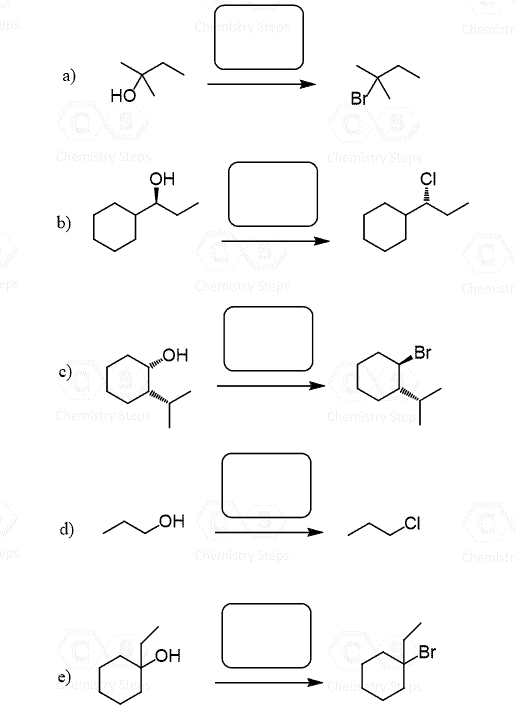
Another strategy for converting alcohols to alkenes can be first converting them to alkyl halides and then doing a Zaitsev or Hofmann elimination. The idea here is again converting the OH into a good leaving group but instead of protonating, we substitute it with a halogen.
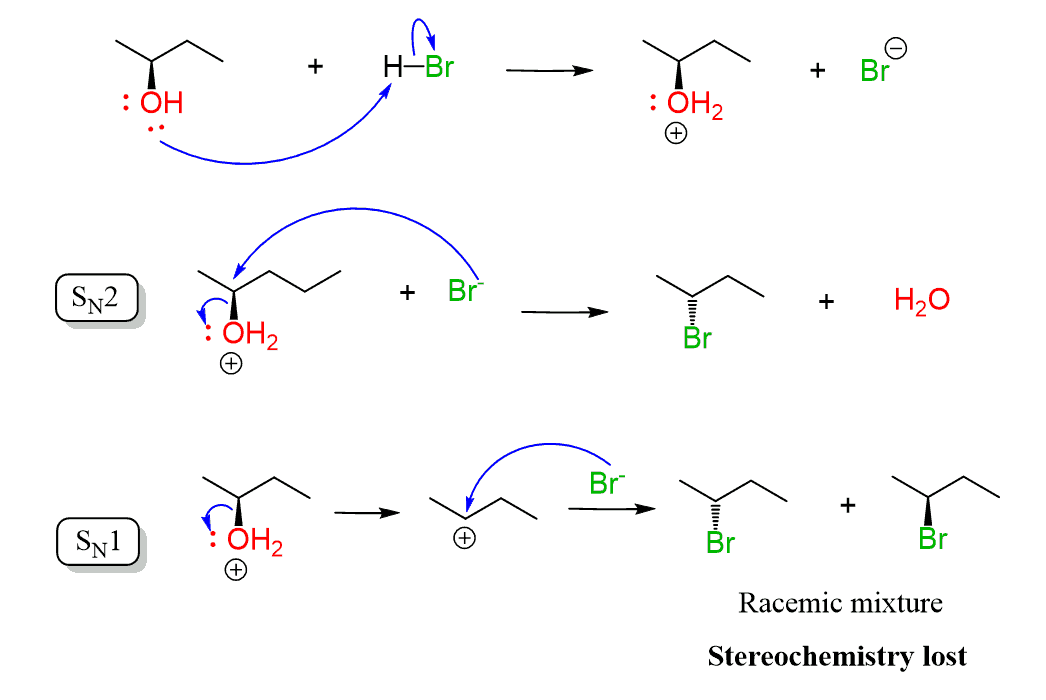
The conversion of secondary alcohols to alkyl halides goes by both SN1 and SN2 mechanisms, and therefore, you’d still need to watch out for possible rearrangement.
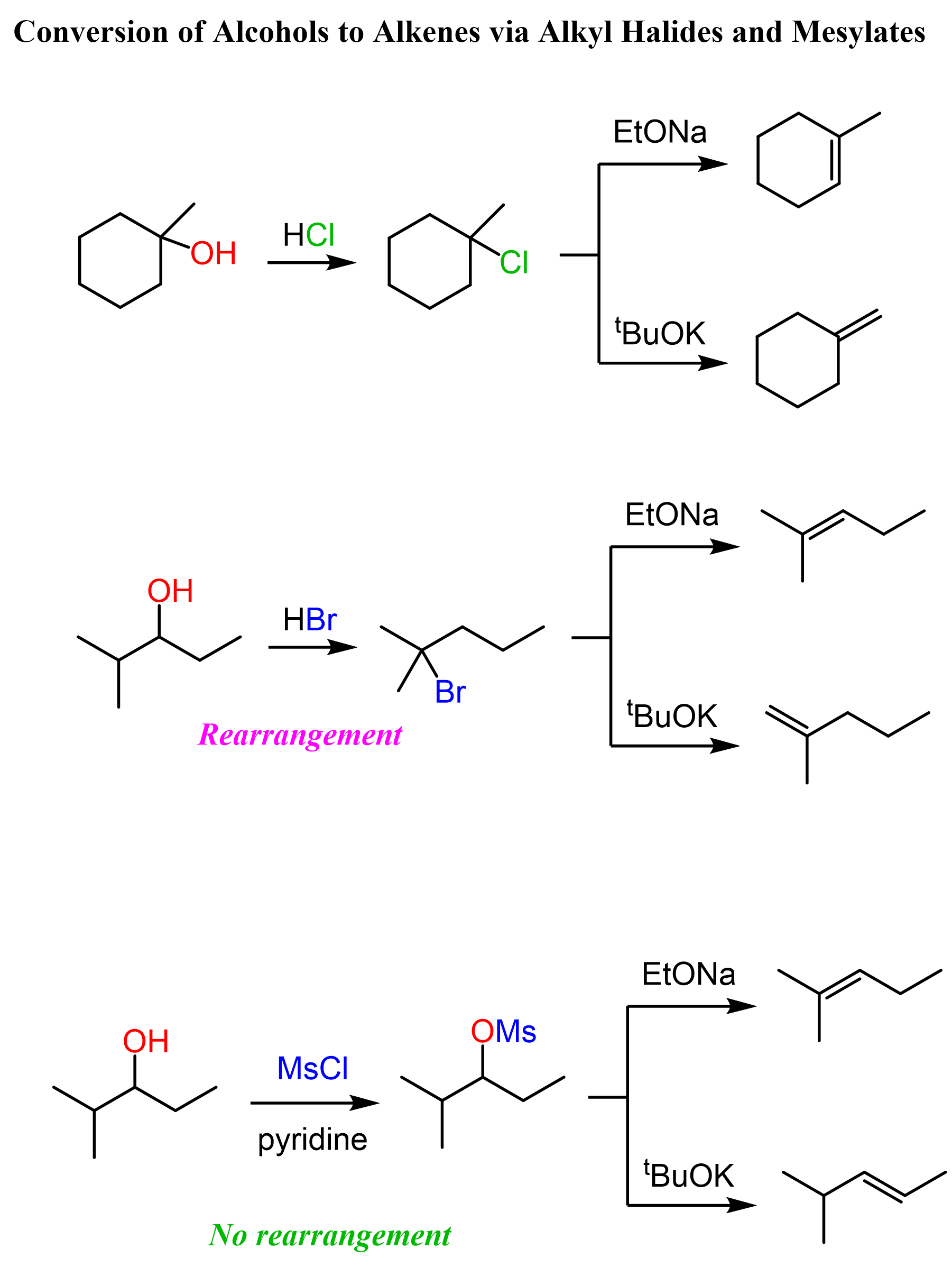
The conversion of the secondary alcohol to a tertiary alkyl halide is an example of a rearrangement. A workaround to avoid this is converting the OH into a mesylate or a tosylate and then doing a Zaitsev or Hoffman elimination. See the last example above, and for more details about converting alcohols to alkyl halides, mesylates, or tosylate, check the corresponding linked articles.
Another approach for converting alcohols to alkyl halides which avoids rearrangements and goes by SN2 mechanism is the use of SOCl2 and PBr3.
The disadvantage of this method is that SOCl2 and PBr3 do not work for tertiary alcohols:
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- POCl3 for Dehydration of Alcohols
- Dehydration of Alcohols by E1 and E2 Elimination
- The Oxidation States of Organic Compounds
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern, and All of That
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Naming Thiols and Sulfides
- Reactions of Thiols
- Alcohols Quiz – Naming, Preparation, and Reactions