Amines from Alcohols Via Substitution Reactions
Although amines are good nucleophiles, we cannot use them is a direct SN2 reaction to achieve the OH to NH2 conversion because the hydroxide ion is a poor leaving group.
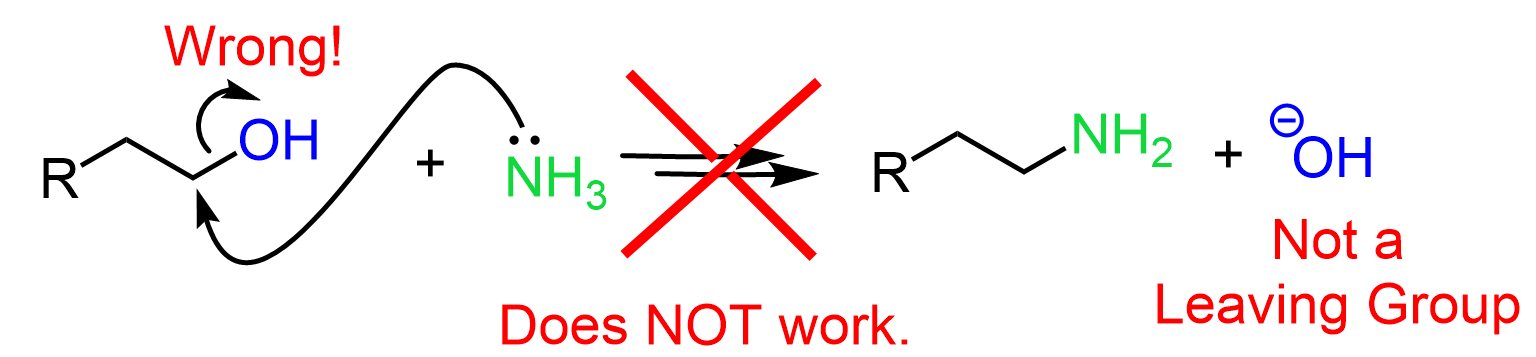
So, the strategy for preparing an amine from an alcohol is first converting the OH into a good leaving group such as a mesylate, tosylate, or a halide, and then reacting the latter with an amine:

Converting alcohols to mesylates or tosylates compared to alkyl halides has some benefits such as controlling the stereochemistry, avoiding rearrangements, and in general avoiding using acidic conditions. You can read about the mechanism and specifics of these methods in the linked articles.
The use of SOCl2 and PBr3 is also another great way to convert primary and secondary alcohols to alkyl halides.
The main drawback of this method is the polyalkylation of the nitrogen nucleophile. The problem is that the amine formed by nucleophilic substitution still has a lone pair of electrons, thus it can serve as a nucleophile or a base reacting with the alkyl halide. This gives a mixture of 1°, 2°, and 3° amines which limits its application.

There are different ways to approach this and talk about them in the article dedicated to the preparation of amines, so feel free to go over that too.
Preparation of Amines via Azides
One way to avoid the alkylation of the primary amine is using an azide salt to convert the R-X alkyl halide or R-Oms/R-OTs intermediate, and then reduce the alkyl azide:
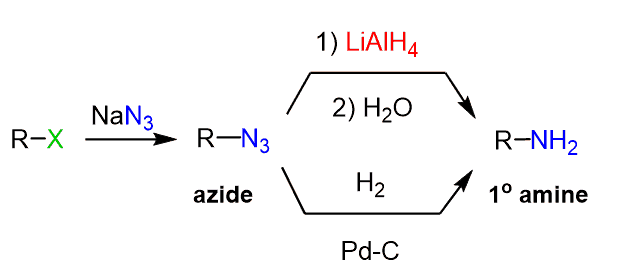
Azide is a good nucleophile and the reaction goes via SN2 reaction so using a polar aprotic solvent such as DMF or DMSO is beneficial.
Preparation of Amines Using Cyanide (–CN) Substitution
Similarly, the cyanide ion can be used as a good nucleophile to convert the alkyl halide to a nitrile which is then reduced to an amino group.
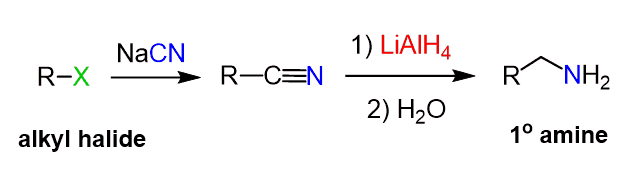
Notice that using the cyanide substitution-reduction method introduces an extra carbon, so be careful in counting the length of the carbon chain.
The Ritter Reaction for Converting Alcohols to Amines
Although the Ritter reaction is not covered in many textbooks, it is a great combination of principles and reactions such as carbocations, loss of a leaving group, nucleophilic addition, and hydrolysis, so you should be good to use it for a variety of functional group transformations without violating what you are allowed to use in your assignments.
It is a nucleophilic addition of a nitrile to a carbocation, followed by the hydrolysis of the C-N triple bond. What is great is that the source of the carbocation can be an alkyl halide, an alkene, and, what is important for this article, an alcohol:
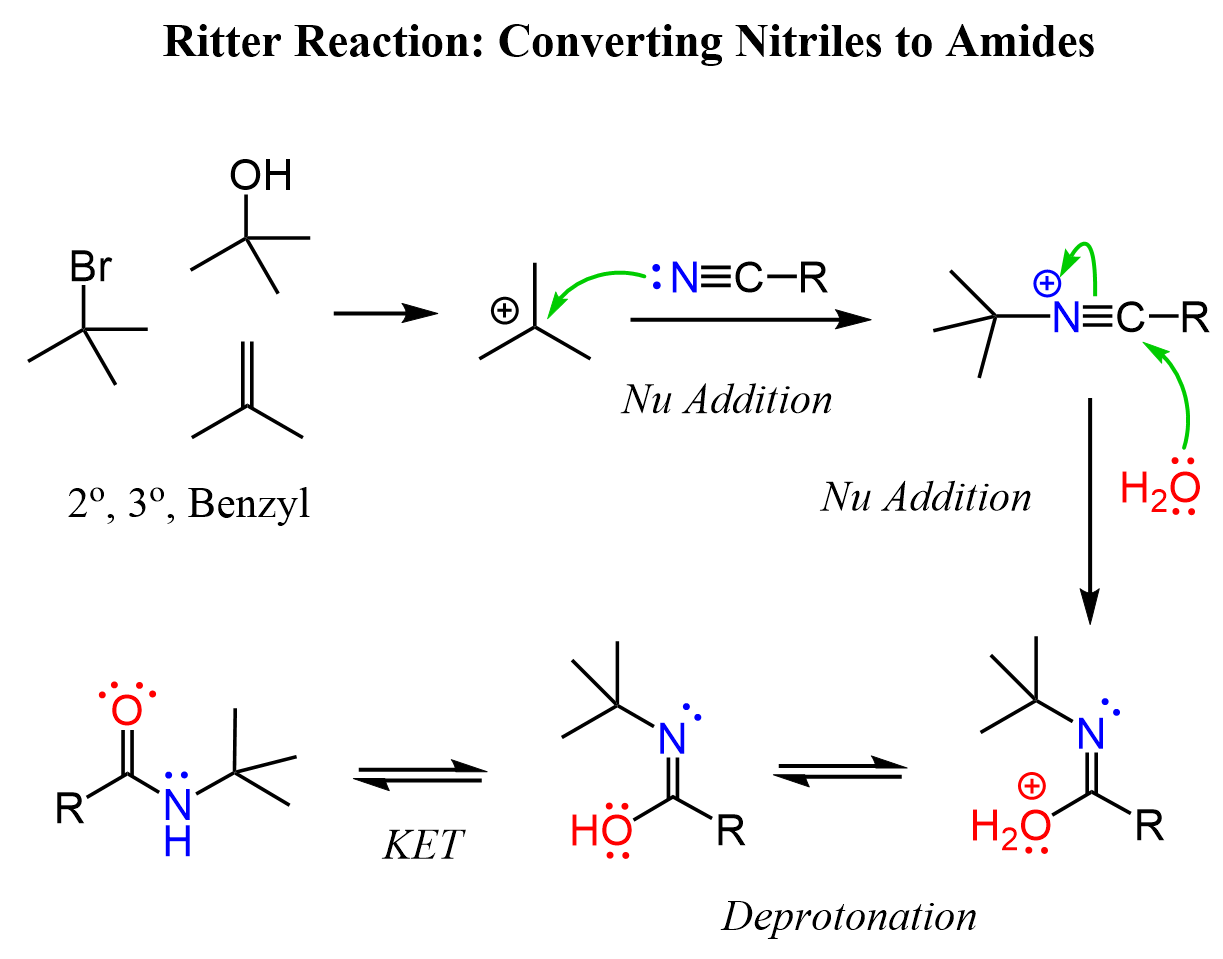
After the nucleophilic attack, the carbon-nitrogen triple bond is activated and a nucleophilic attack of water occurs as we have seen in the hydrolysis of nitriles. The nucleophilic addition of water forms an imidic acid intermediate which tautomerizes to the corresponding amide. What we talked about so far is a nice way of converting, for example, nitriles to amides.
Now, amides can also be hydrolyzed under acidic and basic conditions to the corresponding acid and the amine, so we can construct a summary scheme for converting alcohols to amines using the Ritter reaction.
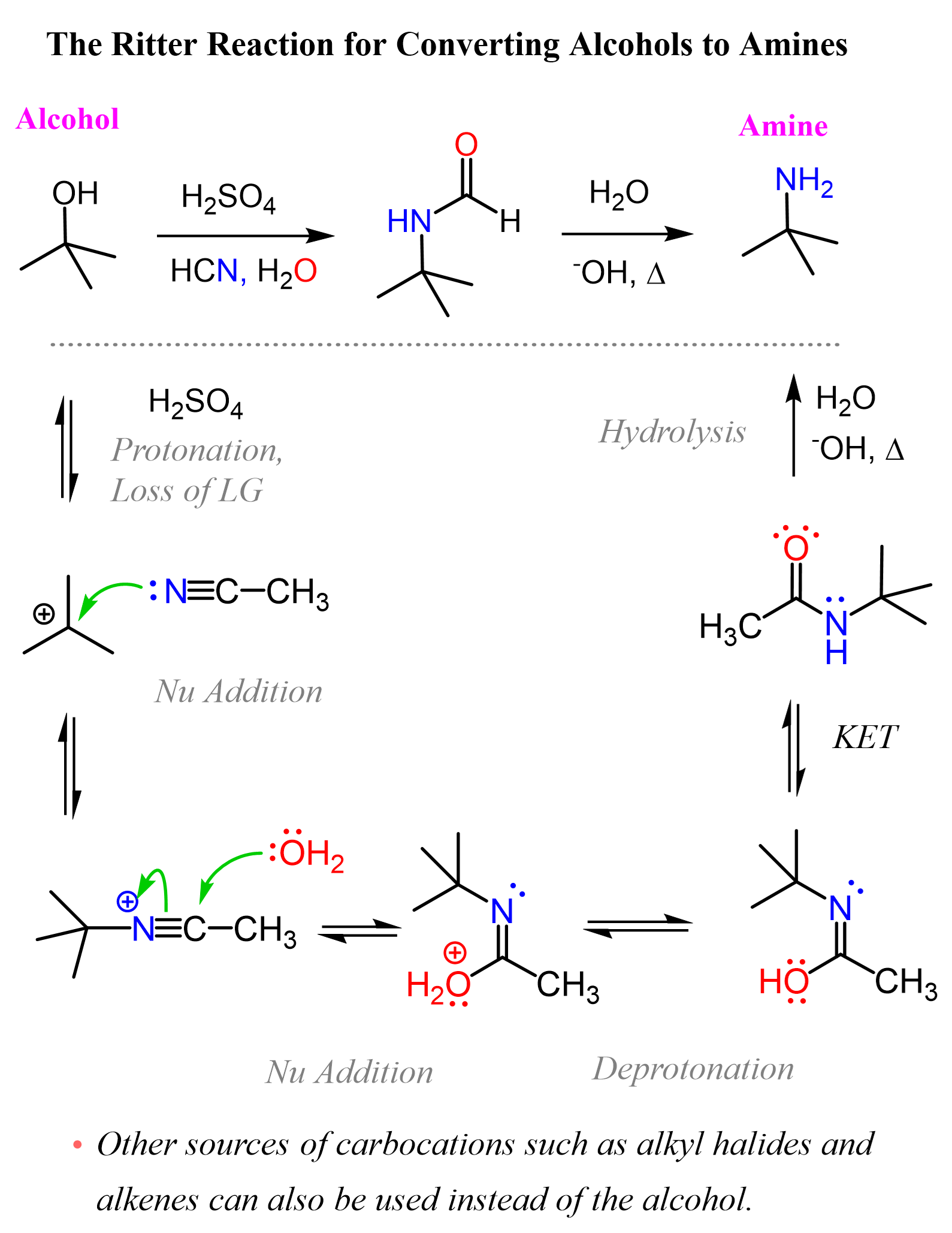
Keep in mind that tertiary and resonance-stabilized alcohols are the best candidates for forming carbocations, but this method will also work with secondary and even primary alcohols when strong Lewis acids are used.
These are the main approaches that come to my mind, and if you can think of more within the undergraduate curriculum, feel free to mention them in the comments section.
More on the relaxations of alcohols can be found in the corresponding articles, practice problems, and the multiple-choice quiz.
Alcohols Quiz – Naming, Preparation, and Reactions
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- POCl3 for Dehydration of Alcohols
- Dehydration of Alcohols by E1 and E2 Elimination
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern and All of That
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Naming Thiols and Sulfides
- Reactions of Thiols
- Alcohols Quiz – Naming, Preparation, and Reactions


