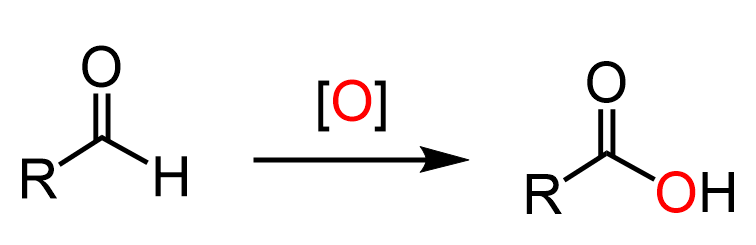
Aldehydes to Carboxylic Acids
Aldehydes can be oxidized to carboxylic acids with a variety of oxidizing agents such as chromic acid, sodium dichromate, potassium permanganate, and sodium hypochlorite.
Here is the summary of oxidizing agents we discussed in the oxidation of alcohols, so feel free to check this article for more about the selectivity and mechanism of each oxidation:

Another way of oxidizing an aldehyde to a carboxylic acid is by using the Tollens’ reagent (Ag+ NH3, HO–):
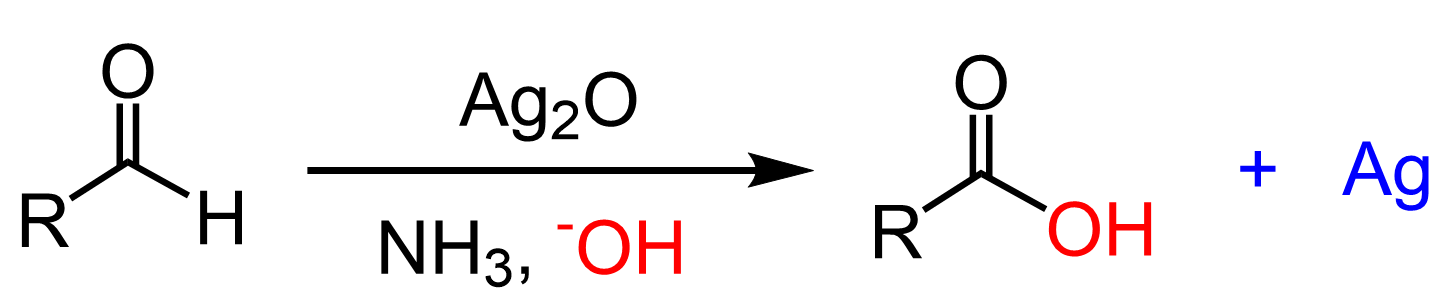
The indicator in Tollens reagent is the by-product Ag which coats the surface of the flask forming a mirror and that is why the reaction is also known as the Tollens test for Aldehyde:

Along with Tollen’s reagent, there is also Fehling’s reagent (Cu2+ in aqueous sodium tartrate), and Benedict’s reagent (Cu2+ in aqueous sodium citrate) that are used for the oxidation of the aldehyde group in carbohydrates:

Ketones to Carboxylic Acids
When thinking about a way of converting a ketone to a carboxylic acid, the first thing that comes to mind is the haloform reaction. It starts with a trihalogenation of the ketone under basic conditions followed by a nucleophilic acyl substitution by addition-elimination mechanism:

While the products of chlorination and bromination are chloroform (CHCl3) and bromoform (CHBr3), respectively, iodination forms a bright yellow solid iodoform (CHI3), which is an indicator for methyl ketones or alcohols that are oxidized to methyl ketones in the presence of iodine. The haloform reaction is mostly known for the reaction of methyl ketones with iodine.
Baeyer-Villiger Oxidation
The Baeyer-Villiger oxidation converts ketones to esters using peroxycarboxylic acids, such as meta-chloroperoxybenzoic acid (mCPBA).

The resulting ester can then be hydrolyzed to the corresponding carboxylic acid under basic or acidic conditions.

The Mechanism of Baeyer-Villiger Oxidations
The first step of the reaction is the activation of the carbonyl group via protonation by the acid followed by a nucleophilic attack by the peroxycarboxylate ion which leads to an intermediate capable of a rearrangement via 1,2-alkyl shift (migration):

The migratory aptitude (R vs R’) of the alkyl groups connected to the carbonyl defines which ester is formed. It has been found that tertiary alkyl groups migrate the fastest followed by secondary and aryl groups: tertiary alkyl > secondary alkyl > aryl > primary alkyl > methyl. For example, we can use the Baeyer-Villiger Oxidations to convert ethyl isopropyl ketone (2-methyl-pentan-3-one) to isopropyl propionate:
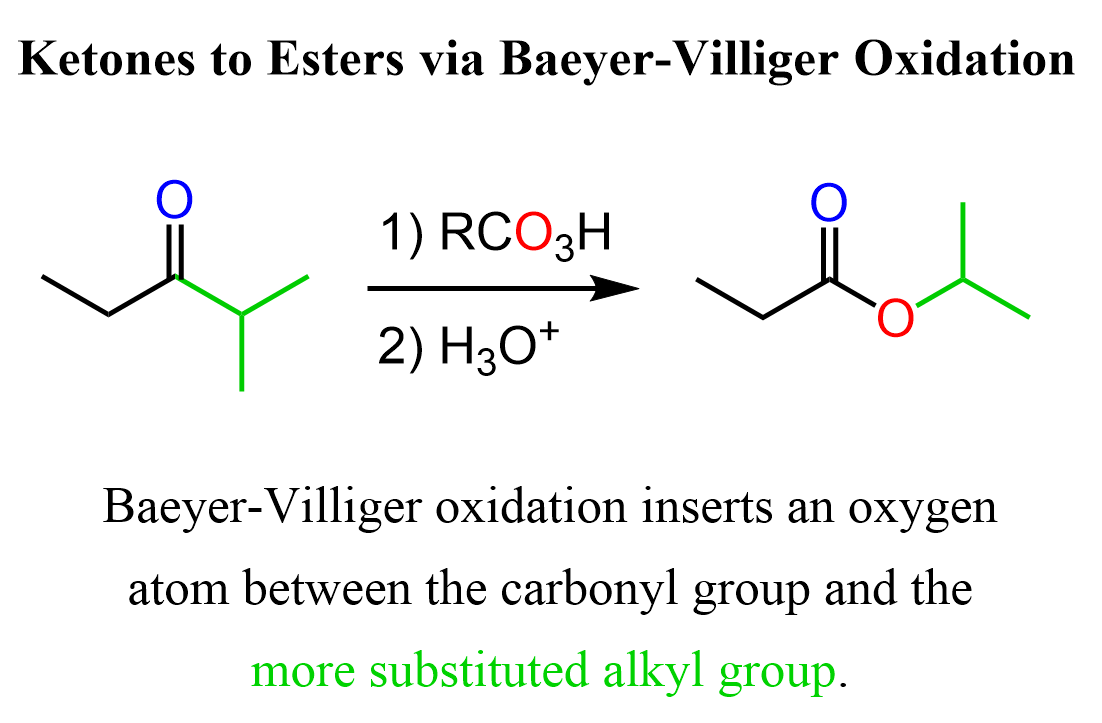
So, for the general pattern of the Baeyer-Villiger Oxidation, remember that it inserts an oxygen atom between the carbonyl group and the more substituted alkyl group.
This has to do with both the electron density and steric considerations, and it is a subject of continuous discussions, which are beyond the scope of this article. For aryl groups, the migratory aptitude increases with the electron density of the ring, so the presence of electron-donating groups makes the rearrangement step faster, which, being the rate-determining step, increases the overall rate of the reaction.
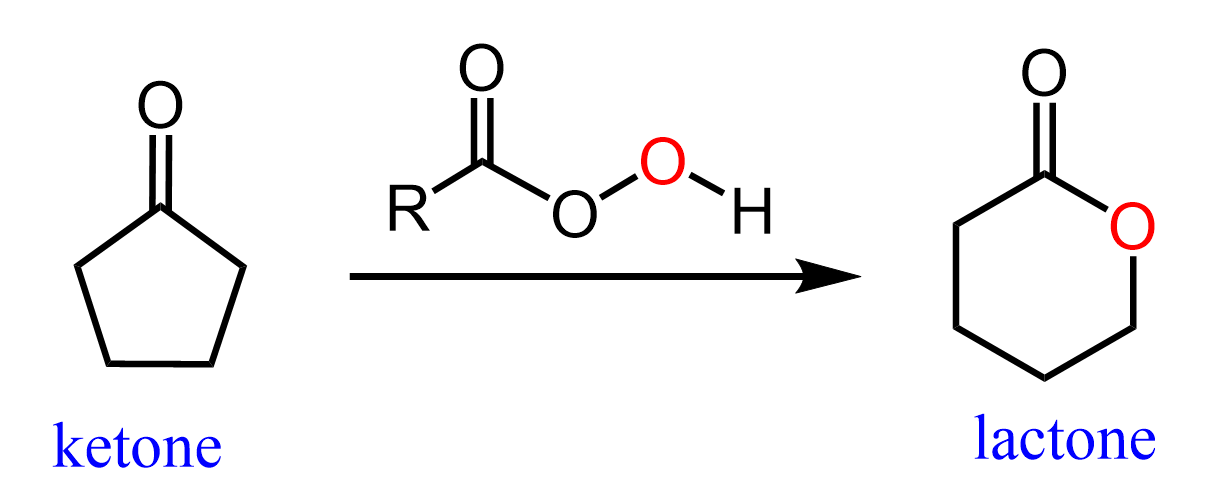
The Baeyer-Villiger Oxidation can also be used for converting cyclic ketones to lactones (cyclic esters):
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- Grignard Reaction with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones

