We have seen, in the halogenation of aldehydes and ketones, that enolates are quite good nucleophiles:

This reaction shows that the ɑ position of a carbonyl compound is a potential nucleophilic site. So, keep this in mind and remember the many occasions where the carbonyl serves as an electrophile because of the polar nature of the C=O double bond.
Examples are all the addition reactions of aldehydes and ketones, the nucleophilic acyl substitutions of carboxylic acid derivatives.
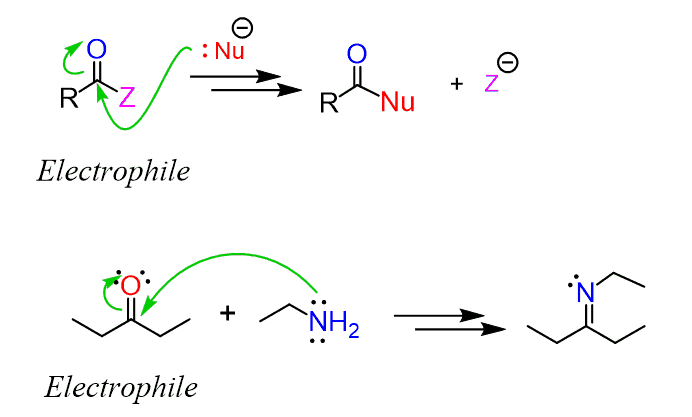
Now, would you expect to happen if we add a base to the solution of an aldehyde? Some of the aldehyde will be converted into an enolate – that’s the first thing, and we now have a mixture of an electrophilic aldehyde and a nucleophilic enolate:
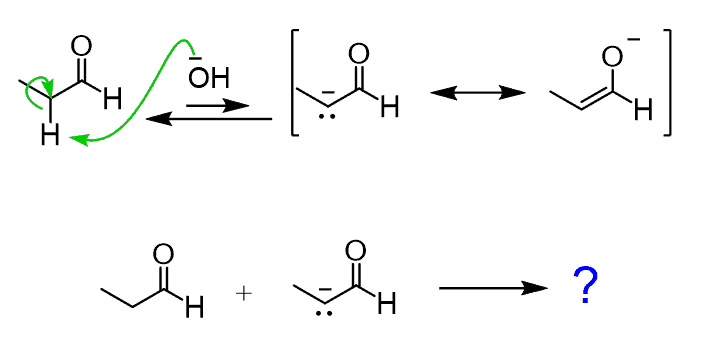
At this point, the enolate is going to attack the electrophilic carbon of the C=O bond. A good analogy to this is the Grignard reaction since in both cases, we have a carbon nucleophile:
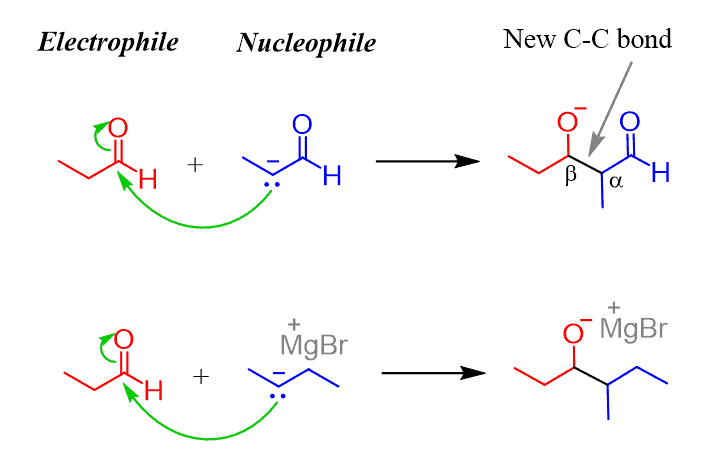
The product of the Grignard reaction is most often an alcohol, while the aldol reaction produces a β-hydroxy carbonyl compound. It is β-hydroxy because the OH group is on the β position of the carbonyl group.
Let’s now put a complete mechanism for the aldol reaction:

Looking at the mechanism, you may wonder why the enolate is shown in a resonance structure with the negative charge on the carbon atom rather than on the oxygen. This is a good question since, we normally expect the negative charge to be on the more electronegative oxygen atom. And the only reason for having it otherwise is to help following the molecules in the reaction easier since it is the carbon atom that acts as the nucleophile.
Ketones in Aldol Reaction
The aldol reaction occurs with ketones as well, however, unlike the aldehydes, the equilibrium here does not favor the aldol product:

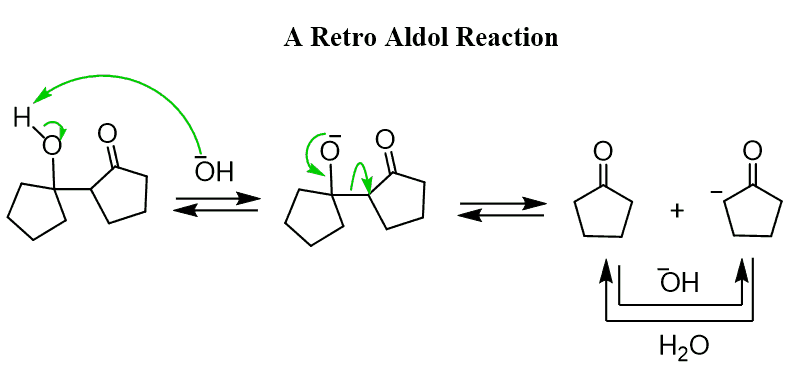
Although there are special distillation techniques to increase the yield of aldol product of a ketone, the reverse reaction of converting the aldol to a ketone is usually faster. This process is called a retro-aldol reaction:
How to Quickly Predict the Aldol Product
Let’s first visualize what comes from where in the aldol product. There are two parts – one from the nucleophilic carbonyl and the other, turned into an alcohol, from the electrophilic carbonyl:
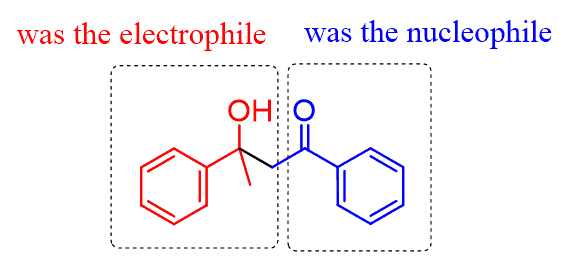
So, what you can do to determine the structure of the aldol is to draw the “V” shape β-hydroxy carbonyl and add the rest of the carbon chain to each side of this unit:

More examples to practice this concept are given here:
Aldol Addition and Condensation Reactions – Practice Problems
What if Different Carbonyl Compounds are Reacted in an Aldol Reaction?
All the examples we discussed were reactions of the same carbonyl compound but what if we have a mixture of, for example, benzaldehyde and acetaldehyde treated with a base?
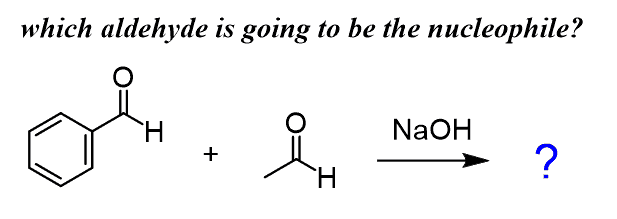
In general, these are called crossed aldol reactions and there are a few scenarios that need to be addressed separately. One of the, shown above, is when one of the carbonyls does not have ɑ hydrogens and, therefore cannot be deprotonated to serve as a nucleophile. So, the aldol product is a result of a nucleophilic addition of the acetaldehyde enolate to benzaldehyde:
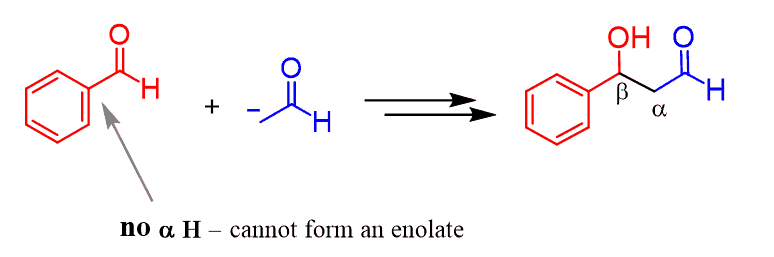
All the other possibilities and details of the crossed aldol reaction will be covered in the following post:
What is the Difference Between the Aldol Reaction and Aldol Condensation?
When the β-hydroxy carbonyl is heated up in the presence of a base, an elimination reaction, called E1CB, occurs and a ɑ, β-unsaturated carbonyl compound is formed:
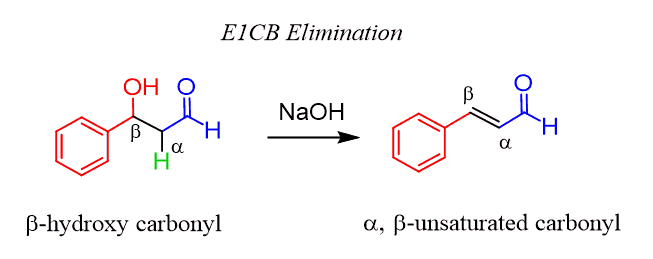
This is known as the aldol condensation reaction the net result of the reaction is adding two molecules together and removing water.
And this particular reaction will occur even at room temperate because the product is a fully conjugated system involving the aromatic ring.
All the details of this Aldol reaction will be covered in a separate post.
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
