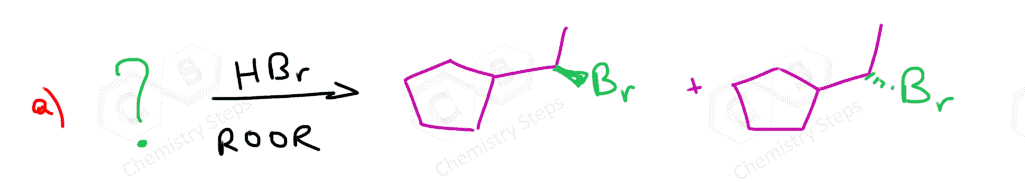
Double bonds are electron-rich and therefore they react with many electron-deficient species, including acids such as HCl, HBr, and HI. The products of these addition reactions of HX acids to alkenes are alkyl halides:

These are hydrohalogenation reactions, which are a type of electrophilic addition reaction to alkenes. The electrophile here is the acid or other molecules that react with the alkene. The alkene can be classified as a Lewis base as it is the electron donor, and it also falls in the category of Brønsted bases because, in these particular reactions, it is also the proton acceptor. We will see, when discussing the mechanism of these reactions, that it is the π bond that acts as a base, and although it is not a strong base, it is still reactive to strong acids.
The Regiochemistry of Hydrohalogenation of Alkenes
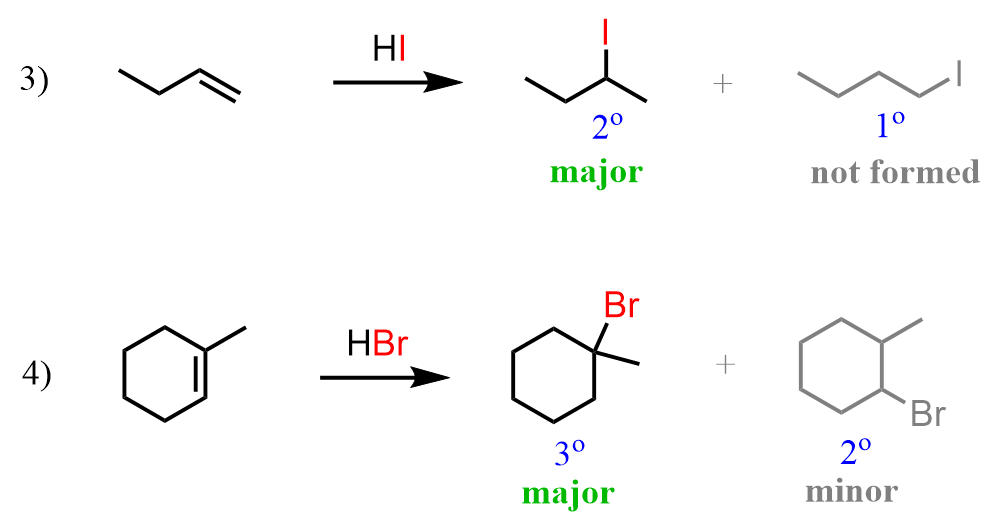
The most important feature of these reactions, applicable in synthesis, is their regiochemistry. Notice how in the third and fourth reactions, the I and Br appeared on the more substituted carbon atoms (secondary in the case of I, and tertiary in the case of Br).
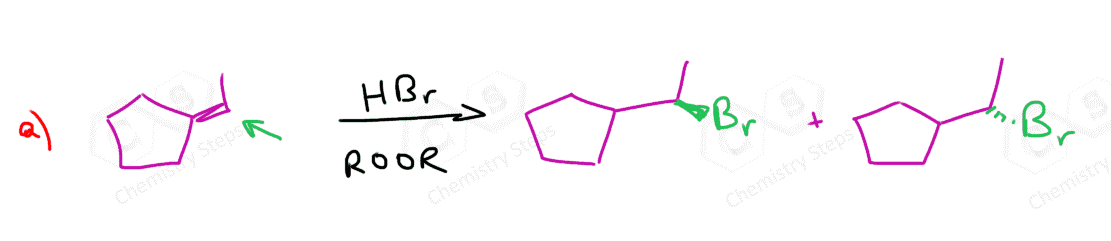
This selectivity can be explained by showing the mechanism of the hydrogen halide additions to alkenes. The rate-determining step of the electrophilic addition of HX acids to alkenes is the first step where the electrons of the π bond attack the hydrogen of the acid, forming a carbocation intermediate. This step defines the product of the reaction as every double bond can be “opened up in two ways, depending on which carbon makes the new C-H bond. For example, in the third reaction, but-1-ene can break the double bond either to the left or to the right, leading to a secondary or a tertiary carbocation:
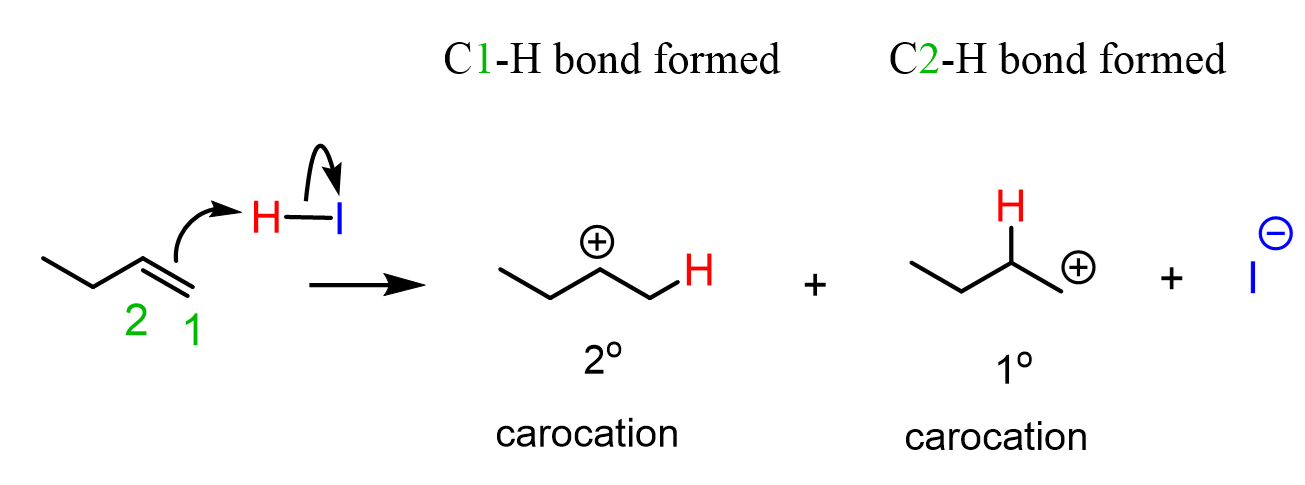
To predict which carbocation is favored, we need to compare their stability. What do you remember about the stability of carbocations?
Because of electron-donating and hyperconjugation, the stability of carbocations increases with the number of alkyl groups connected to the positively charged carbon:
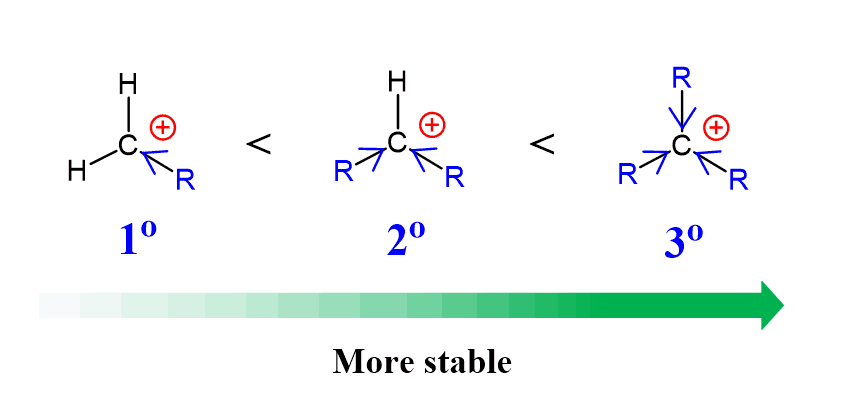
Therefore, the secondary carbocation is the preferred intermediate in this reaction, and, upon formation, it is quickly attacked by the halide anion, giving the corresponding alkyl halide as the final product:
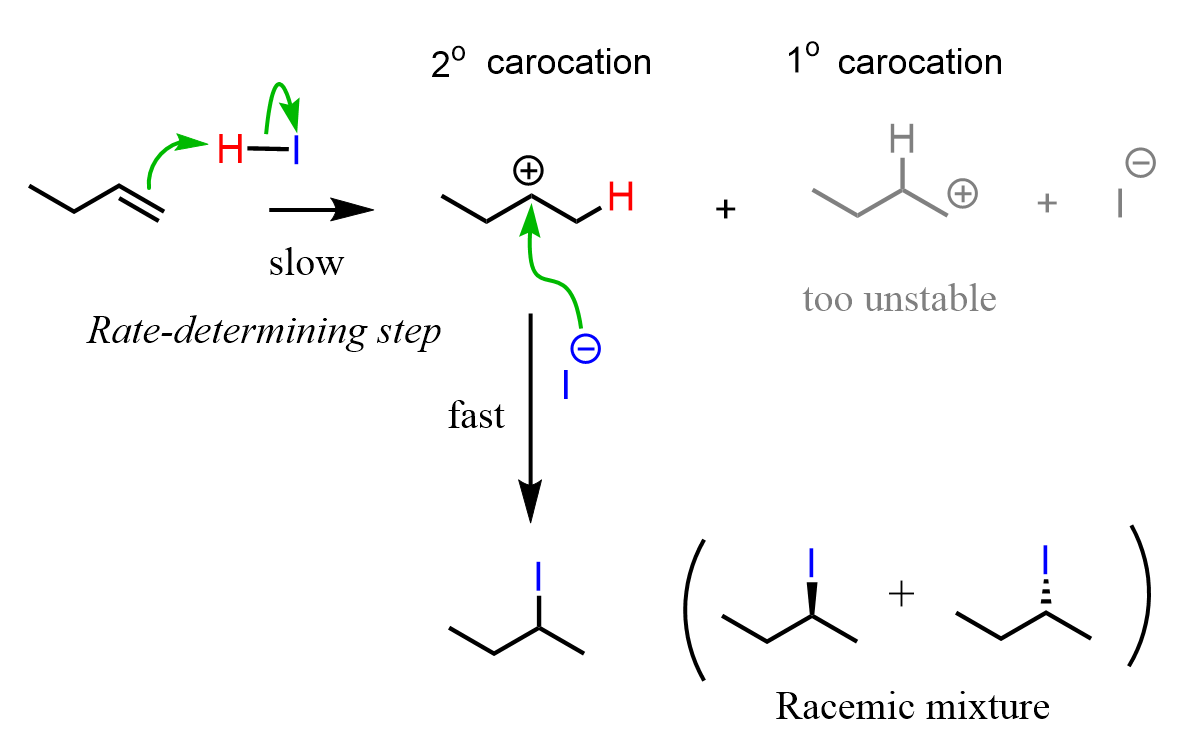
Notice that the product is formed as a mixture of two enantiomers in equal quantities (racemic mixture). This is the stereochemistry of the hydrohalogenation, which we will discuss later in this post.
Let’s also go back a little bit to address the first two reactions:
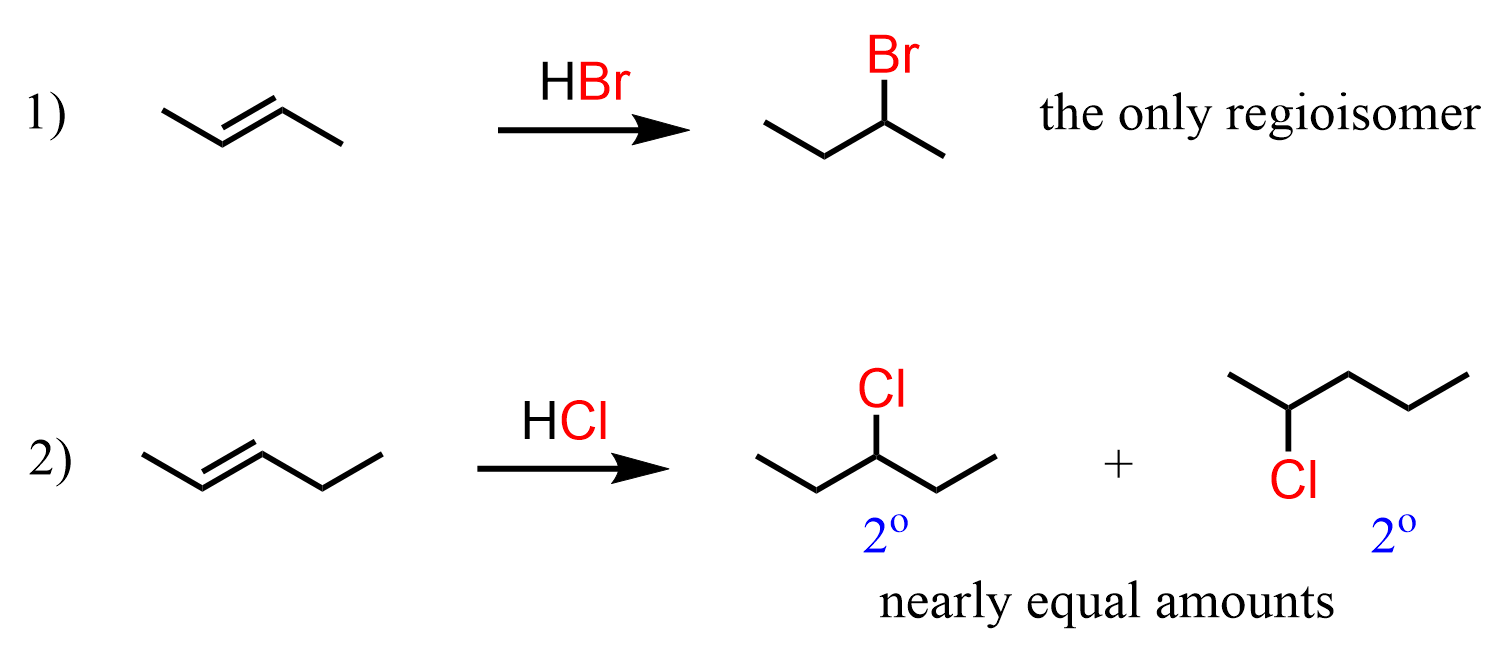
The alkene in the first reaction is symmetrical, and regardless of which carbon the Br adds to, we get the same product. It is a racemic mixture of two enantiomers, as 2-bromobutane is a chiral compound, but there is no regiochemistry in addition reactions to symmetrical alkenes.
In the second reaction, we have pent-2-ene, and it is not symmetrical. On one end of the double bond, there is a methyl and on the other end, there is an ethyl group. And even though methyl is a smaller group than ethyl, and one may expect to see some preference for Br adding to the less sterically hindered carbon, little or no regioselectivity is observed in hydrogen halide additions to alkenes with different sizes of alkyl groups.
To summarize the regiochemistry of HX addition reactions to alkenes, remember that the halogen adds to the more substituted carbon of the double bond. We can also say that the hydrogen adds to the less substituted carbon of the double bond, or which the same to the one that already has more hydrogens. This is known as Markovnikov’s rule, and we are going to see it quite often in the reactions of alkenes.
Rearrangements in Hydrohalogenation of Alkenes
The formation of carbocation intermediates brings the possibility of rearrangements during hydrohalogenation of alkenes, just like we have seen in SN1 and E1 reactions.
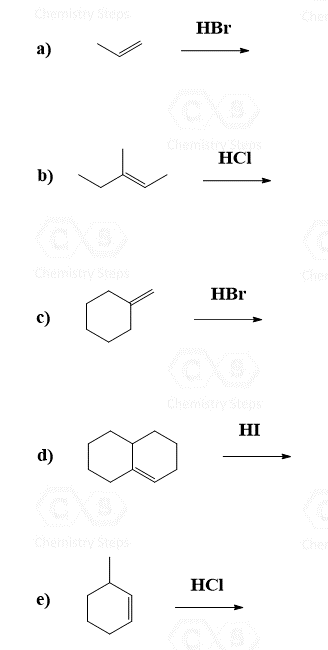
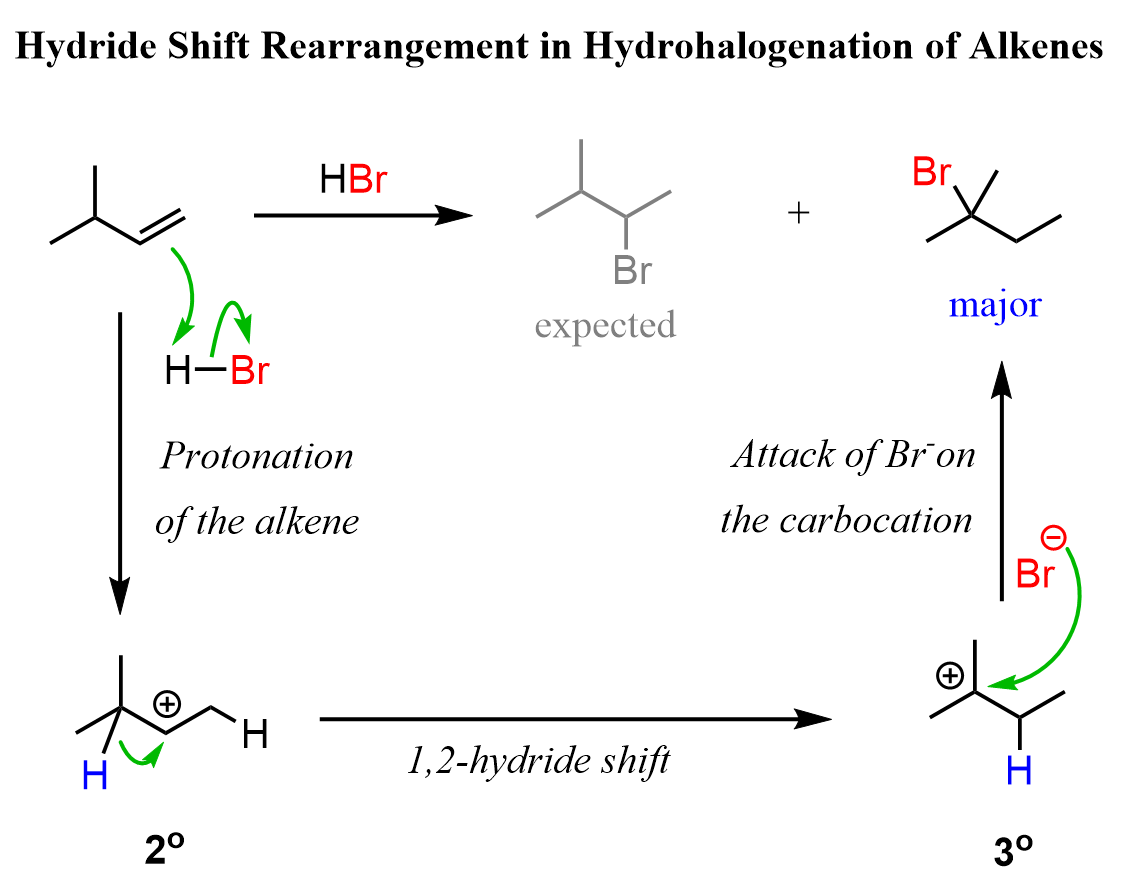
For example, when the following alkene is treated with HBr, based on Markovnikov’s rule, we may expect the Br to add to the middle carbon because of the greater stability of the secondary carbocation:
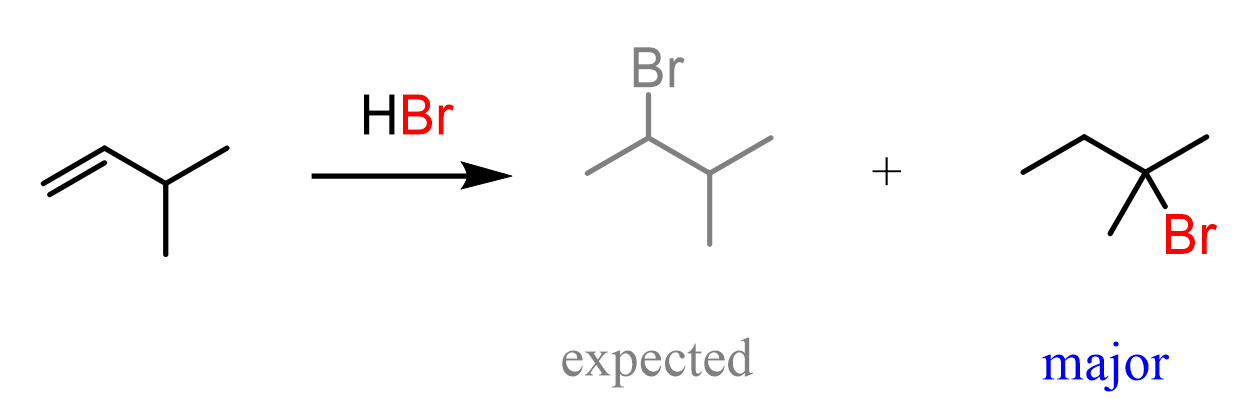
So, how does this happen? In the first step, we know that the hydrogen addition will form a secondary carbocation because it is more stable than the possible primary carbocation. This is true; upon protonation, the secondary carbocation is formed; however, before reacting with the bromide ion, it undergoes a 1,2-hydride shift rearrangement to form a more stable tertiary carbocation:
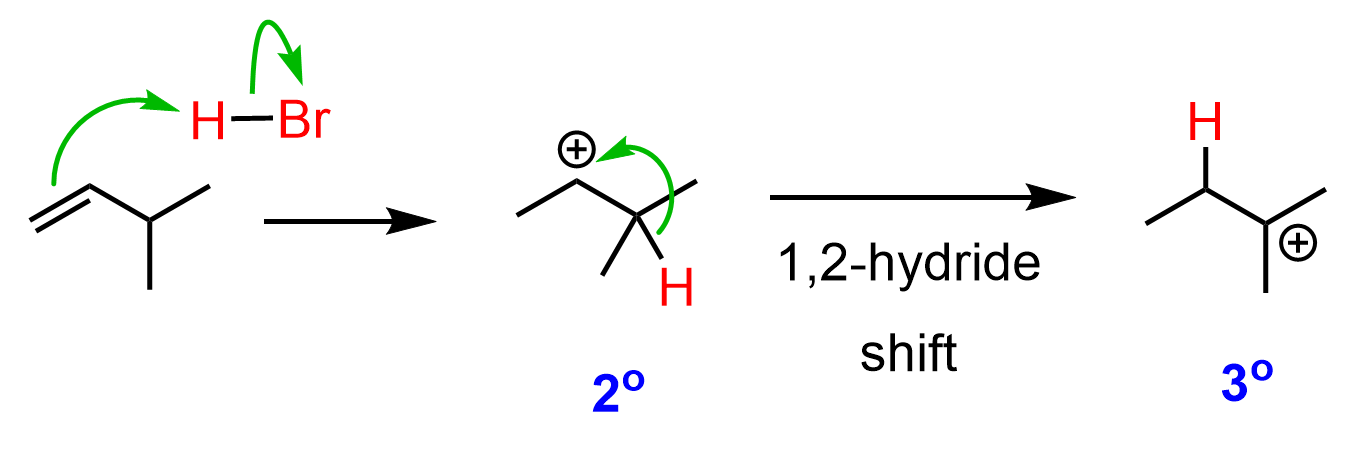
Once the tertiary carbocation is formed, it is attacked by the bromide, resulting in a tertiary alkyl halide as the major product of the reaction:
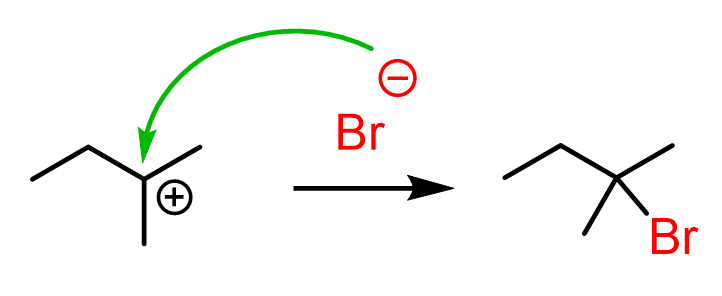
Let’s combine the steps in one scheme to represent the mechanism of hydride shift rearrangement in the hydrohalogenation of alkenes:
Rearrangements during hydrohalogenation of alkenes, and other reactions going via carbocation intermediates, can also happen via 1,2-methyl (more accurately methide) shifts. For example, instead of the hydrogen, there was a methyl group on the alkene we discussed above, a 1,2-methyl shift would occur to transform the secondary carbocation to a more stable tertiary carbocation:
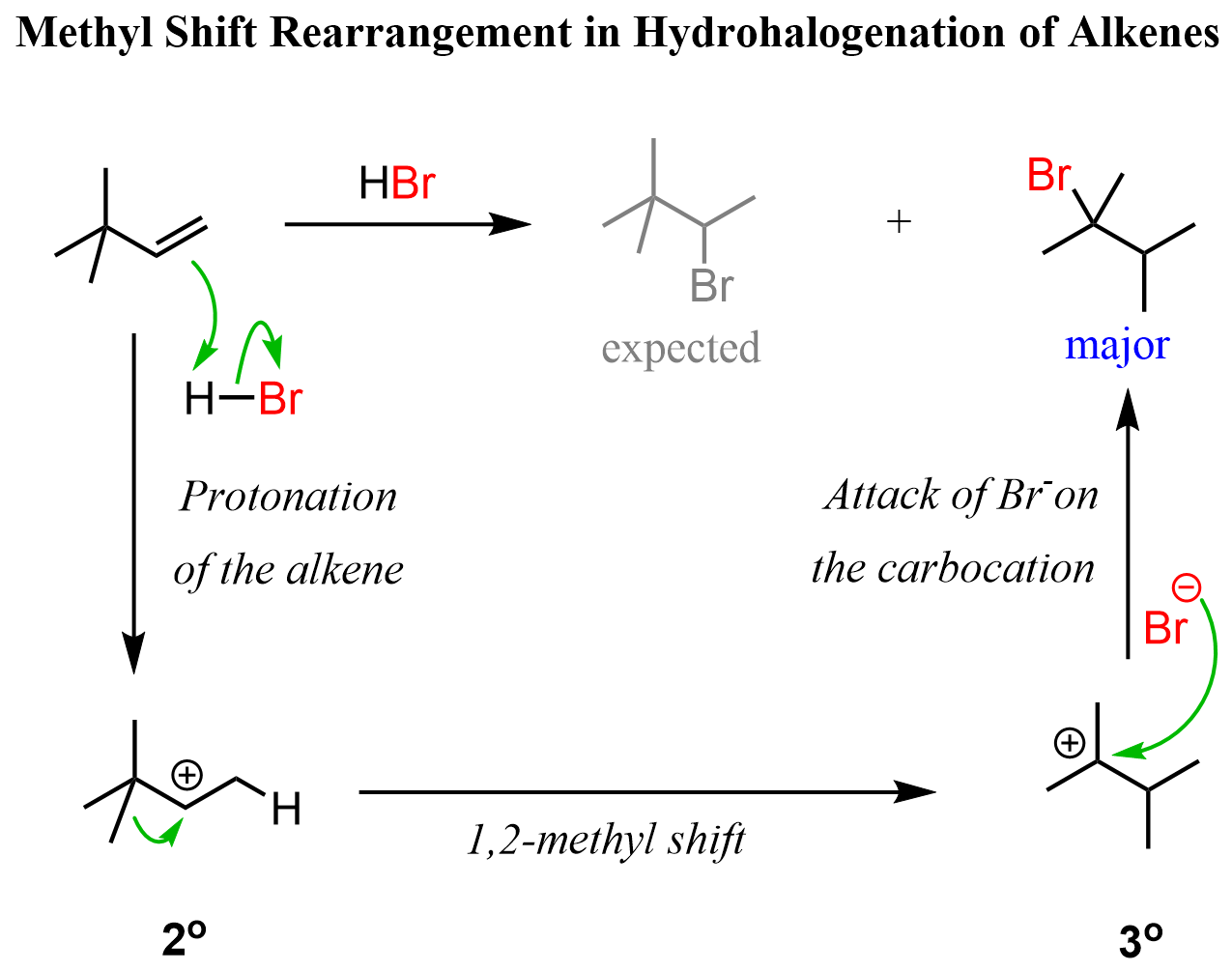
To summarize this section, remember that, when possible, rearrangements are going to happen. Therefore, you need to keep them in mind when looking at any reaction involving a carbocation intermediate. In a typical undergraduate organic chemistry course, these are going to be SN1, E1, and the electrophilic addition reactions to alkenes, such as those of hydrohalic acids (hydrogen halides), and acid-catalyzed hydration.
The Stereochemistry of Hydrohalogenation of Alkenes
There is no stereoselectivity in the addition of acids to alkenes. Let’s see what products are formed when the alkene has no chirality centers.
Hydrohalogenation of Alkenes with No Chirality Centers
For example, in the following addition reaction of HCl to the unsymmetrical alkene, we obtain a mixture of enantiomers, which means the reaction is not selective to any of these stereoisomers:
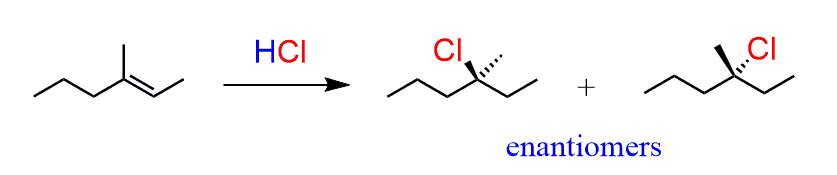
- To summarize, addition reactions of alkenes with no stereogenic center that form a product with one stereogenic center produce a racemic mixture of enantiomers.
This is explained by the fact that carbocations are sp2-hybridized, flat centers (we are talking about the positively charged carbon), and the nucleophilic attack occurs from both sides:
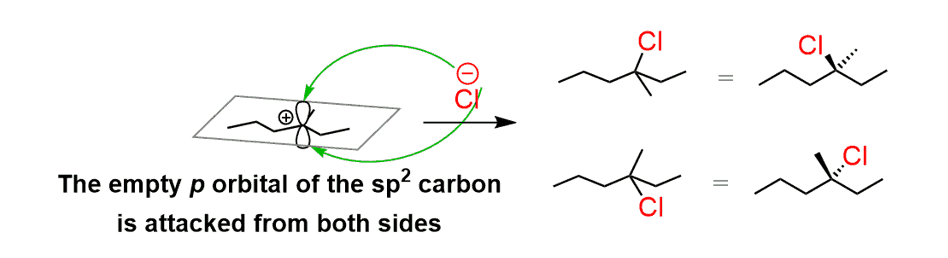
This attack happens in the same amounts, and as a result, a racemic mixture of two enantiomers is obtained.
Hydrohalogenation of Alkenes with a Chirality Center
If the starting alkene contains a chirality center, and the addition to the double bond creates a new chirality center, then the products are diastereomers:

The asymmetric center in the starting material is not changed since it does not participate in the reaction. The new asymmetric center, however, is opposite for each product, depending on the face the bromide had attacked the carbocation.
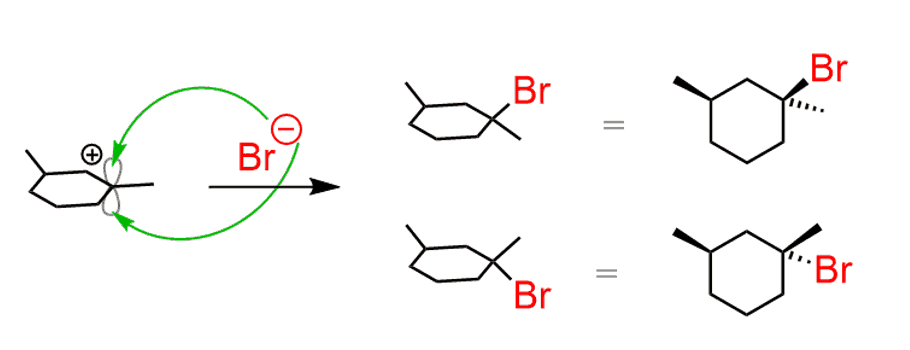
- Therefore, the products are a mixture of diastereomers. Similar to this, SN1 reactions can also produce diastereomers, even though we usually say that they give a racemic mixture. You can check problems 3.5 and 3.6.
Addition Reactions that Form a Product with Two Chirality Centers
Let’s consider the reaction of 1,2-dimethylcyclohexene with HBr. The starting material does not have any asymmetric centers. However, it produces four stereoisomers in this reaction!

Let’s see how this happens and what the relationship is between these stereoisomers. The first step is, as usual, the protonation of the double bond, and what is important here is to remember/visualize that the H can add from both faces of the double bond:

Notice that because of the hydrogen addition from different faces, the new chirality center can be either R or S, and statistically, it forms in a 50:50 ratio. There is no preference as to hydrogen addition from one side or the other side – no stereoselectivity.
Similar to this, once the carbocation is formed, the bromide ion attacks the positively charged, trigonal planar carbon from above or below. This variety of additions results in four stereoisomers as final products.

These are pairs of enantiomers, and each enantiomer has two diastereomers:

There is no control over the stereochemistry of this reaction, and the proton, as well as the bromide (or any other substituent), adds from both sides in equal amounts. Therefore, the reaction is not stereoselective, i.e., none of the stereoisomers is formed preferentially. It is also not stereospecific, as the same products are obtained regardless of whether the cis or trans stereoisomers of the alkene are used.
- Therefore, when two stereogenic centers are formed in an addition reaction of alkenes, all the possible stereoisomers are formed, and the product is a mixture of enantiomers and diastereomers.
Syn and Anti Additions to Alkenes
Labeling the addition as “Above” and “Below’ is not scientific and not accurate either as the direction depends on the viewer. Therefore, a more universal approach is used to describe the stereochemistry of additions to the double bond. When two groups add to the same side of the double bond, it is called a syn addition and when they add from different sides, it is an anti addition.

The hydrohalogenation of alkenes occurs both via syn and anti addition.
A representative example of a syn addition to alkenes would be the hydroboration-oxidation reaction, where the H and OH groups are added to the same side of the double bond. The reaction, however, is going with a different mechanism; there are no ionic intermediates, and it is a concerted mechanism:
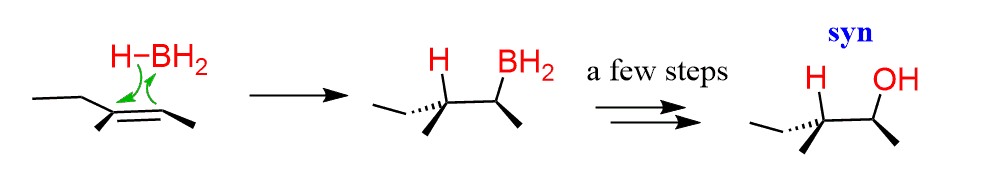
A good example of an anti-addition is the dihydroxylation reaction by peroxy acids.

Notice how the H and OH groups appear on opposite sides in the product.