Both alkynes and alkenes are unsaturated hydrocarbons, and this unsaturation is the result of having a π bond in the molecule. One π bond is one degree of unsaturation, as the molecule can add one hydrogen molecule, and two π bonds indicate two degrees of unsaturation. Therefore, we can say that alkynes are more unsaturated than alkenes, which are between alkanes and alkynes. Therefore, the complete reduction of alkynes to alkanes proceeds via the formation of an intermediate alkene, and very often, it is difficult to stop the reduction at this stage.

So, keep in mind that typical catalytic hydrogenation of alkynes in the presence of Pd or Pt catalysts reduces them all the way to alkanes, so let’s first talk about these.
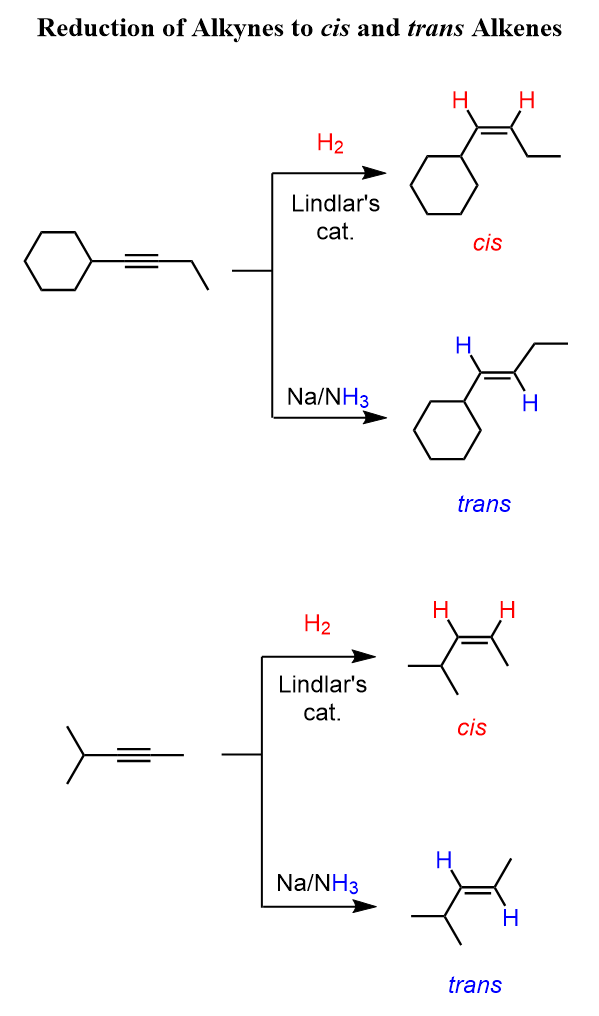
Reduction of Alkynes to cis Alkenes (Lindlar Catalyst)
Depending on the catalyst used, we can selectively convert alkynes to cis and trans isomers. Recall that, unlike alkynes, alkenes are not linear, and when unsymmetrical, they can be cis or trans.
To stop the reduction of alkynes at the alkene stage, we need to reduce the reactivity of the catalyst. That’s where Lindlar’s catalyst comes in. This is still a palladium catalyst, but it is deactivated with CaCO₃ and quinoline.

It delivers one equivalent of H₂ and stops at the cis alkene, again by syn addition.

Another option is P-2 catalyst (nickel boride), which also gives cis alkenes from alkynes.

Reduction of Alkynes to trans Alkenes
The catalytic reduction of alkynes does not allow for preparing (E)- or trans-alkenes as the hydrogen adds in syn geometry. Trans alkenes are prepared by reducing alkynes using Na or Li in NH₃. The reaction goes through a radical mechanism, and the hydrogen is added in an anti-fashion.

The reaction starts with an electron transfer from lithium (or sodium) atoms to the triple bond of the alkyne, forming a radical anion, which deprotonates ammonia. The resulting radical picks up another electron from the metal atom, becoming a carbanion, which is then protonated by ammonia.
To summarize the three variations of reducing alkynes, remember that catalytic hydrogenation with Pd or Pt fully reduces the alkyne to an alkane via the addition of two equivalents of H₂.
Using Lindlar’s catalyst or P-2 catalyst allows partial reduction to a cis alkene, via syn addition, by limiting the reactivity of the catalyst.
For trans alkenes, the dissolving metal reduction with Na or Li in liquid ammonia is used, which proceeds through a radical mechanism and gives anti addition of hydrogen.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Introduction to Alkynes
- Naming Alkynes by IUPAC Nomenclature Rules – Practice Problems
- Preparation of Alkynes by Elimination Reactions
- Hydrohalogenation of Alkynes
- Addition of Water to Alkynes
- Acid-Catalyzed Hydration of Alkynes with Practice Problems
- Reduction of Alkynes
- Halogenation of Alkynes
- Hydroboration-Oxidation of Alkynes with Practice Problems
- Ozonolysis of Alkynes with Practice Problems
- Alkylation of Terminal Alkynes in Organic Synthesis with Practice Problems
- Reactions of Acetylide Ions
- Alkyne reactions summary practice problems
- Alkyne Synthesis Reactions Practice Problems
- Alkyne Naming and Reactions Practice Quiz
- Reactions Map of Alkynes

