Alkynes and nitriles are structurally similar, so let’s discuss how we can replace one of the carbons in the alkyne with a nitrogen:
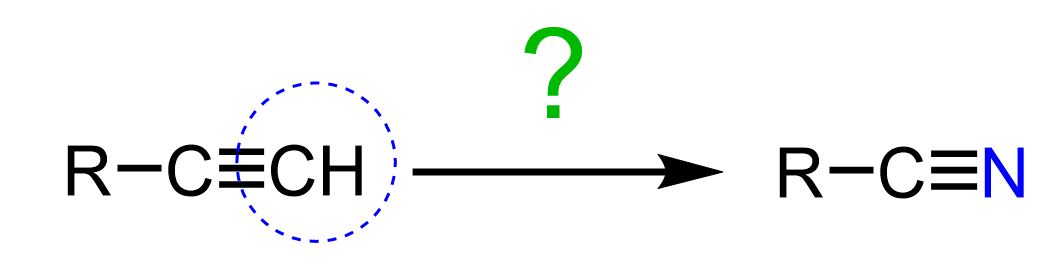
When one of the carbons is replaced, the carbon chain will be shortened by one. However, we may also need to keep the number of carbons or increase it by one, therefore, we will address scenarios for the conversion of alkynes to nitriles:
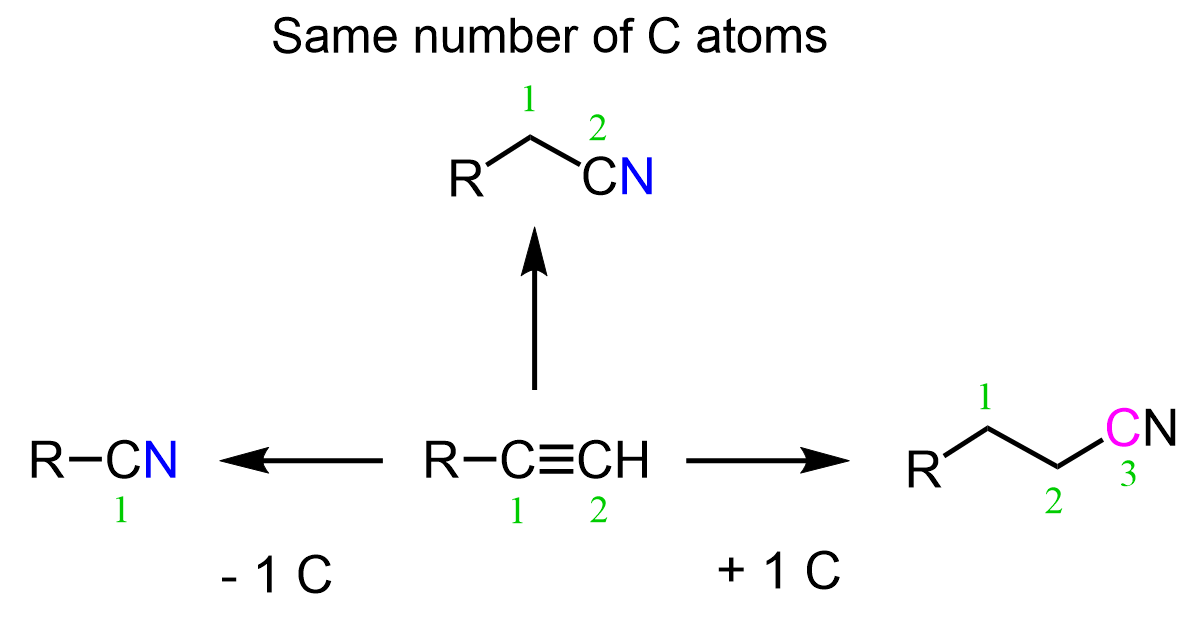
Alkynes to Nitriles: -1 Carbon
Nitriles are carboxylic acid derivatives, so we can first cleave the carbon-carbon triple bond by a strong oxidizing agent forming a carboxylic acid. After this, we convert the carboxylic acid to a primary amide using a coupling agent such as EDC or DCC, and in the last step, we use a dehydrating agent such as SOCl2, POCl3, or P2O5 to obtain the nitrile:
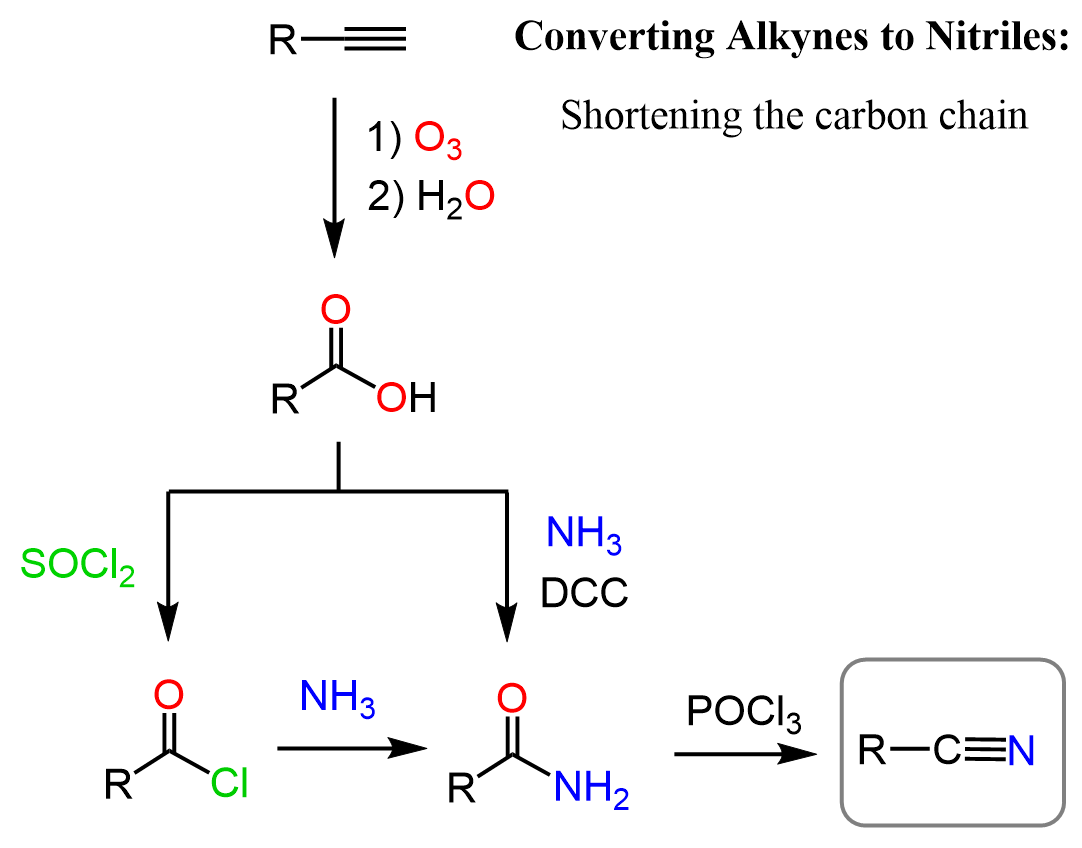
The coupling reactions of carboxylic acids with amines are at times challenging too, so the alternative of converting the acid to acid chloride first is also shown in the synthetic scheme. Recall that acid chlorides are the most reactive derivatives of carboxylic acids, and they readily react with all types of nucleophiles including amines.
Alkynes to Nitriles Retaining the Carbon Chain
The nitrile functional group contains a carbon, therefore, to keep the number of carbons unchanged, we can remove one from the alkyne and reintroduce it with the nitrile. In the strategy discussed above, we shortened the carbon chain by an oxidative cleavage of the triple bond. So, here, we can reduce the resulting carboxylic acid to a primary alcohol, then substitute the OH group with a nitrile by first converting it into a good leaving group. This can be done with an HX acid, mesylation or tosylation:
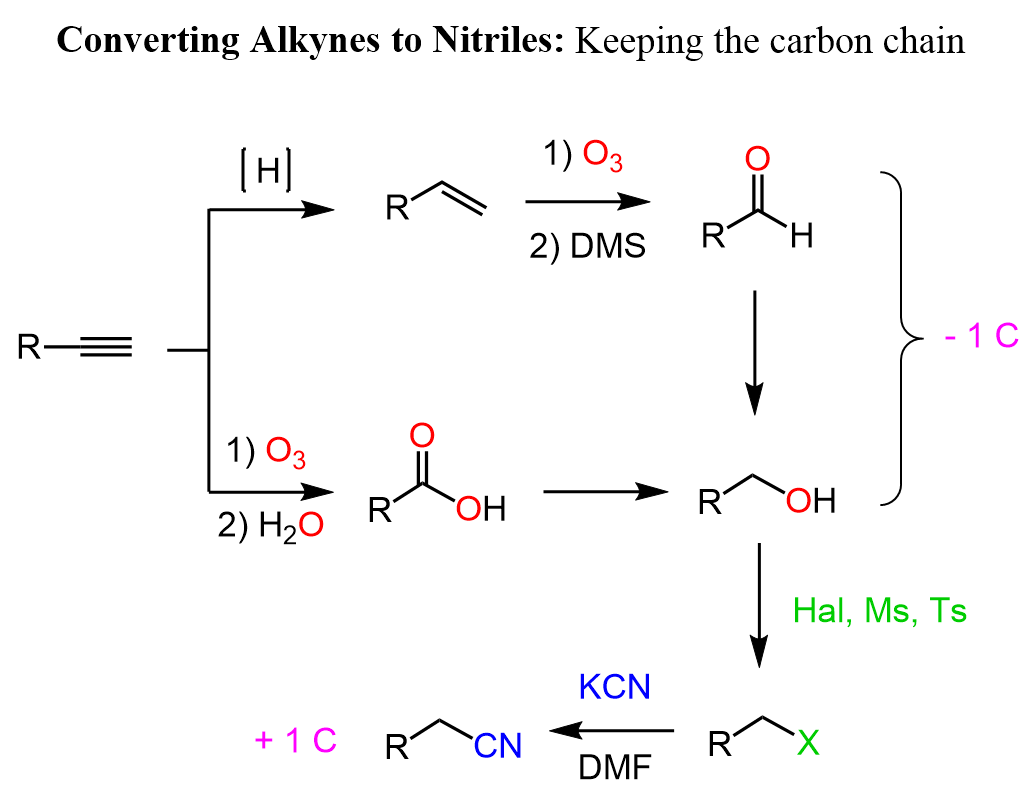
Notice that we can also reduce the alkyne to an alkene first and cleave the double bond to an aldehyde or an acid and then reduce it to the primary alcohol.
Alkynes to Nitriles: +1 Carbon
To add an extra carbon, we can prepare a primary alcohol via hydroboration-oxidation of the alkyne, convert the OH into a good leaving group, and do an SN2 substitution with sodium cyanide:
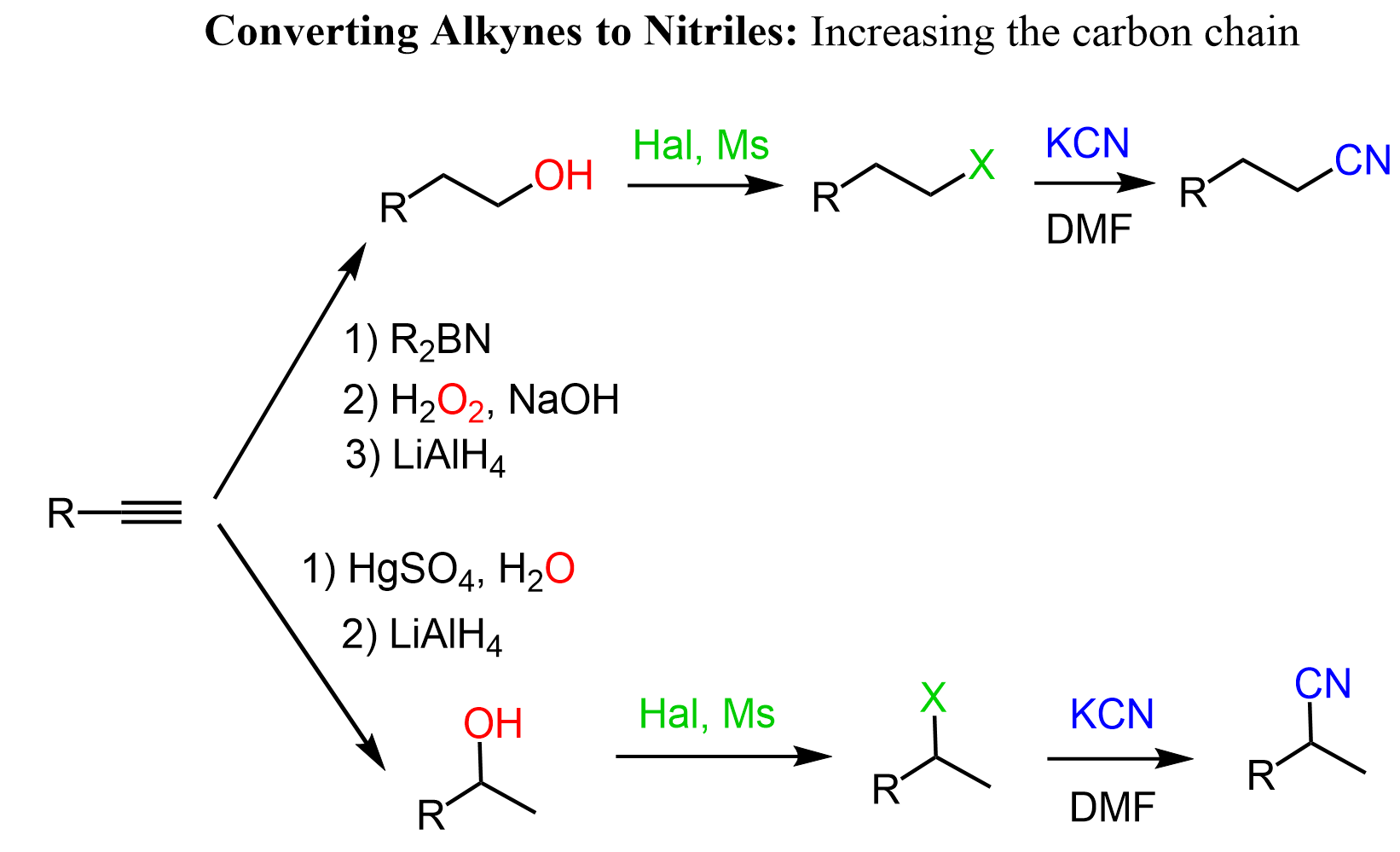
The second path is a conversion of the alkyne into a secondary nitrile via Markovnikov hydration of the alkyne followed by a substitution of the OH group with CN. Recall that the addition of water to alkynes forms an aldehyde or a ketone, therefore, LiAlH4 is used to reduce it to an alcohol.
Internal Alkynes to Nitriles
To convert an internal alkyne to a nitrile, we can oxidize the triple bond to two carboxylic acids, convert the acid into an amide, and dehydrate the amide to a nitrile:
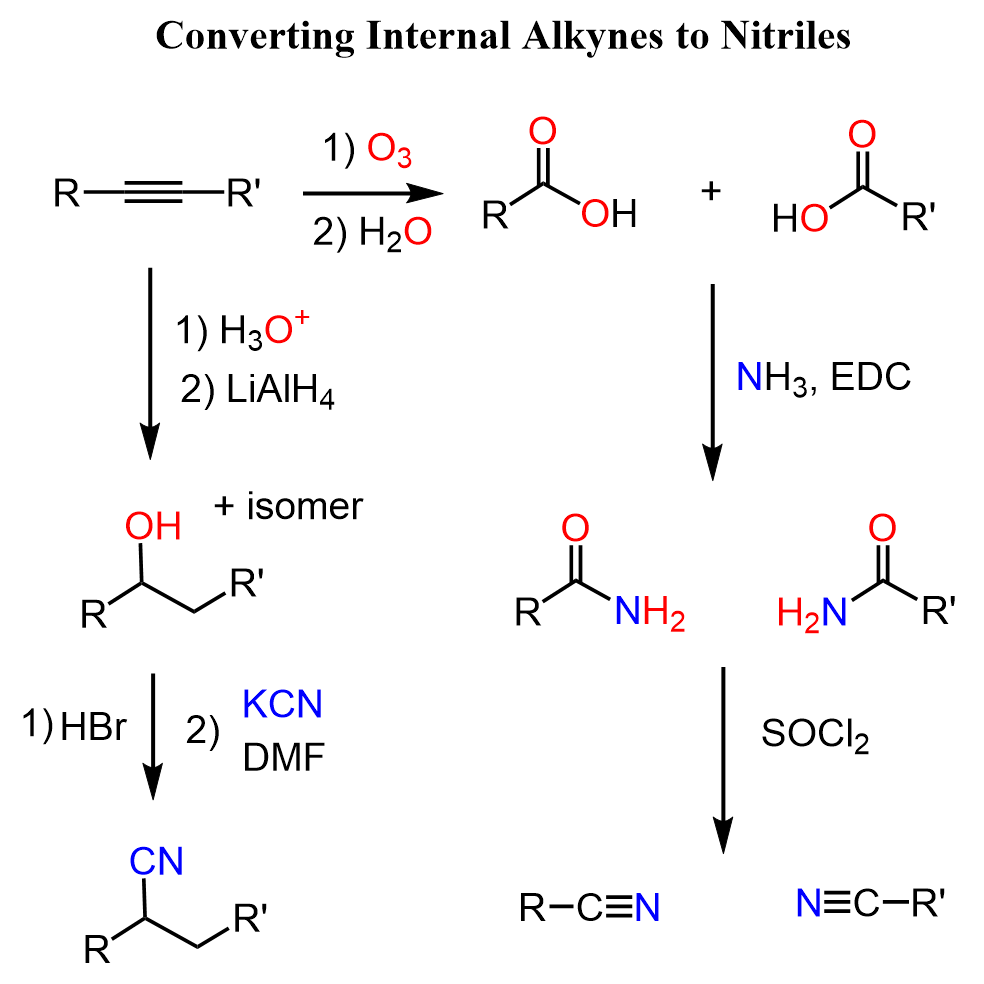
To add a nitrile to the triple bond, we can hydrate the latter to an alcohol and substitute the OH with the CN group.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Introduction to Alkynes
- Naming Alkynes by IUPAC Nomenclature Rules – Practice Problems
- Preparation of Alkynes by Elimination Reactions
- Hydrohalogenation of Alkynes
- Addition of Water to Alkynes
- Acid-Catalyzed Hydration of Alkynes with Practice Problems
- Reduction of Alkynes
- Halogenation of Alkynes
- Hydroboration-Oxidation of Alkynes with Practice Problems
- Ozonolysis of Alkynes with Practice Problems
- Alkylation of Terminal Alkynes in Organic Synthesis with Practice Problems
- Alkyne reactions summary practice problems
- Alkyne Synthesis Reactions Practice Problems
- Alkyne Naming and Reactions Practice Quiz

