Base-Catalyzed Hydrolysis of Amides
Amides are the least reactive derivatives of carboxylic acids because the conjugate base of an amine, formed in a nucleophilic substitution reaction, is a poor leaving group.
For example, in the reaction with a hydroxide, we are comparing the leaving group ability of the hydroxide ion with the conjugate base of the amine:

Comparing the pKa values of water (15.7 or 14 according to recent debates – J. Chem. Educ. 2017, 94, 6, 690–695), and amines (38), we know that the hydroxy group is a much weaker base and therefore, a better leaving group than a conjugate base of an amine.
Therefore, this reaction is not expected to work. However, with a large excess of the hydroxide and excessive heating, amides can be hydrolyzed to carboxylate salts.
The base-catalyzed hydrolysis of amides starts with the nucleophilic addition of the –OH to the carbonyl group. In the next, extremely unfavorable elimination step (step 2), the conjugate base of the amine is kicked out from the tetrahedral intermediate:
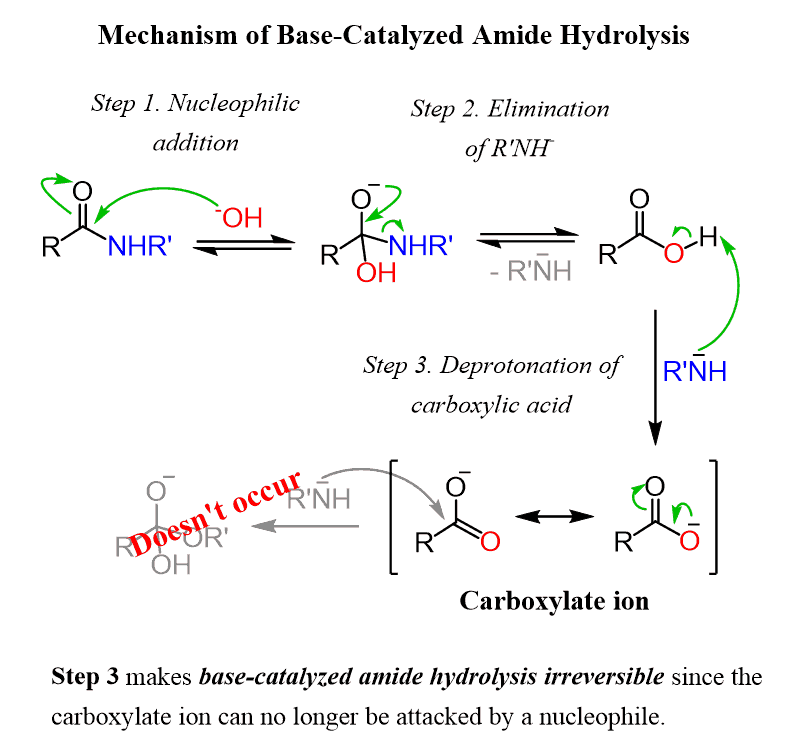
The reaction is driven to completion in a quick, irreversible deprotonation of the carboxylic acid by the newly formed RNH–.
Acid-Catalyzed Hydrolysis of Amides
The acid-catalyzed hydrolysis of amides follows the same steps as we have seen in the hydrolysis of esters. The only difference is the change of the leaving group from ROH to RNH3+.

In both cases, heat and an excess of reactants are needed to push the reaction forward:

Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives

“we know that the hydroxy group are much weaker bases and therefore better leaving groups than a conjugate base of an amine” This is not worded effectively. Since the conjugate acid of a hydroxyl ion, H2O, has a higher pKa, this means that it is a weak acid. The conjugate base of a weak acid is considered strong, unless it is compared to a weaker acid/stronger conjugate base pair.
I think rephrasing would be helpful to distinguish that it’s not an ABSOLUTE fact that hydroxy groups are weak bases/better leaving groups in all cases.
There is some excessive wording or confusion here…
First, you took only part of the sentence which reads ” Comparing the pKa values of water (15.7) and amines (38), we know that the hydroxy group is a much weaker base and therefore better leaving group than a conjugate base of an amine. It is still mentioned that we are comparing it with the conjugate base of an amine, and it is absolutely correct that OH- is a better leaving group than, for example, RNH- or the conjugate base of any amine.
You mentioned, “Since the conjugate acid of a hydroxyl ion, H2O, has a higher pKa, this means that it is a weak acid.” Higher than what? What are we comparing it to?
“it’s not an ABSOLUTE fact that hydroxy groups are weak bases/better leaving groups in all cases”. Nobody said that here. In fact, hydroxyl is a poor leaving group, and that is what we mention all throughout substitution and elimination reactions. A few cases when it leaves by itself without being converted into a good leaving group such as Ms, Ts, Tf, or H2O, are the E1CB elimination, and reactions leading to tetrahedral intermediates which expel it as it is a better leaving group than RNH- or R-.
Let me know if you have questions.