Have you seen your professor talking about BH₃ and H₂O₂ and not quite understood what it is and when to use it?
Ok, so, keeping it simple, we use BH₃ together with a peroxide to place an OH group on the less substituted carbon of the double bond.
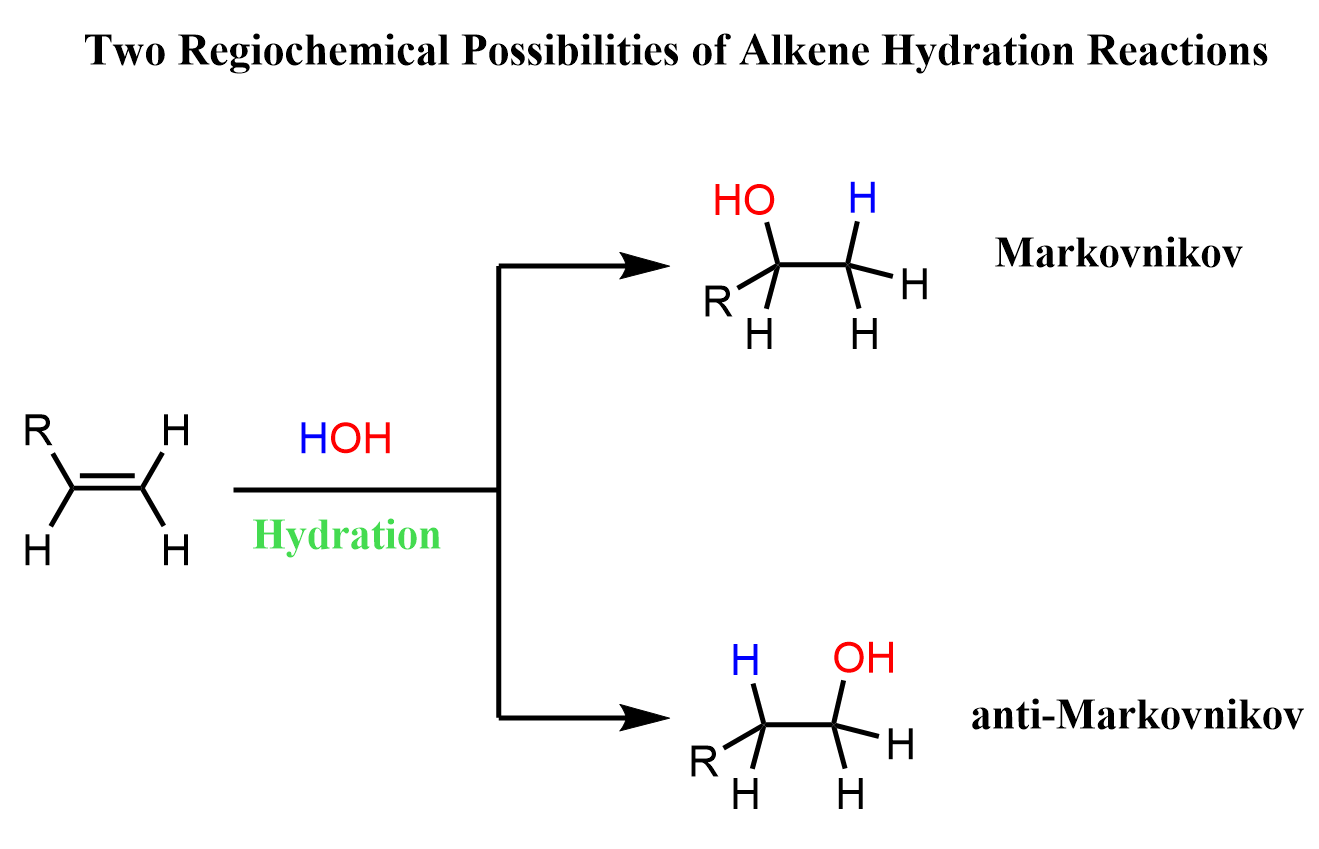
Now, it is not just adding an OH to the double bond, right? – We are talking about a hydration reaction of alkenes. We are actually adding an H and OH to the two carbons of the double bond.
If we remember Markovnikov’s rule, it says that in the addition of HX (X = Halogens or OH), the hydrogen adds to the less substituted carbon, and X, in this case OH, to the more substituted carbon atom.
So, the BH₃ + H₂O₂ combination is used for a hydroboration-oxidation reaction, which is an anti-Markovnikov addition of water to alkenes and alkynes.
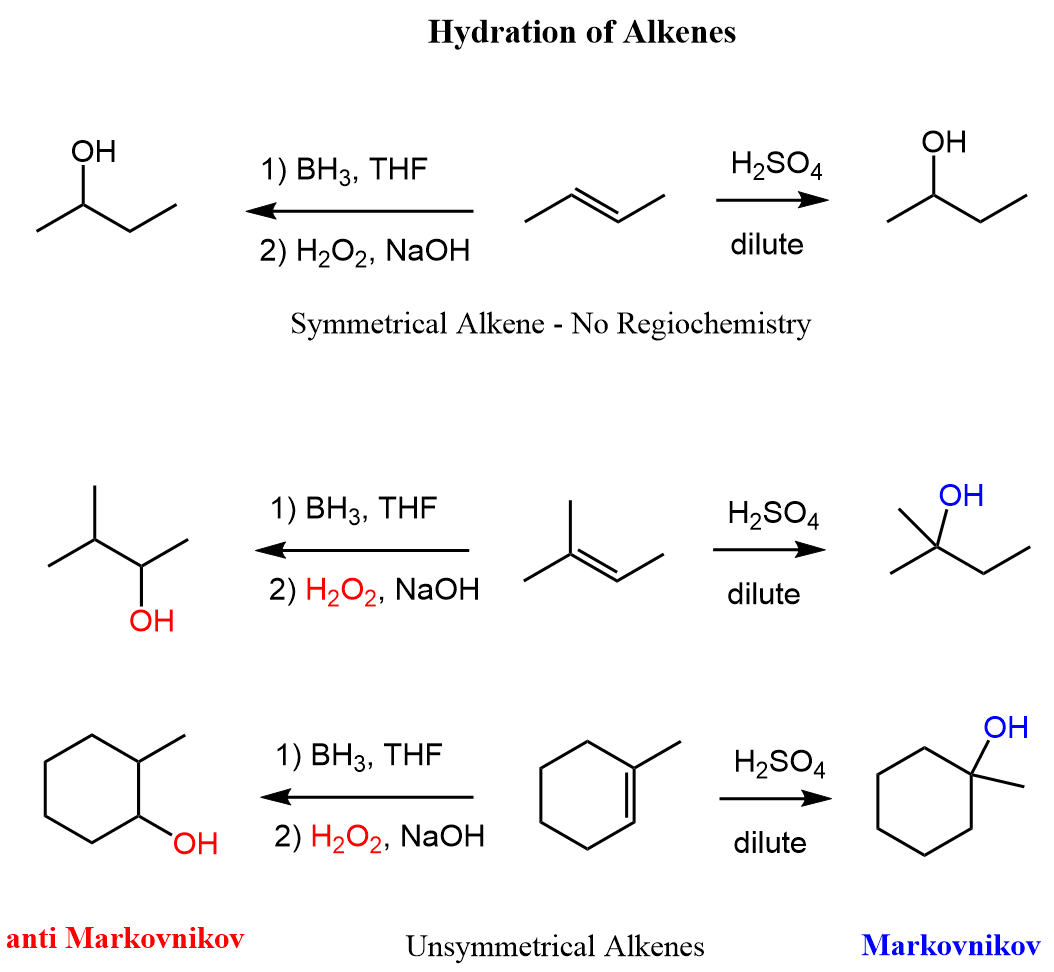
Here are some examples, comparing the Markovnikov and anti-Markovnikov hydration of alkenes:
The Mechanism of Hydroboration-Oxidation of Alkenes
BH₃ is a Lewis acid as boron has an empty p orbital. Alkenes and the epoxide, on the other hand, are electron-rich species; we have the π electrons and the lone pairs on the oxygens. The role of the boron here is to act as a Lewis acid and bring the alkene and the peroxide together. Once on the same boron atom, these two then react by an alkyl shift that ultimately forms the C–O bond.
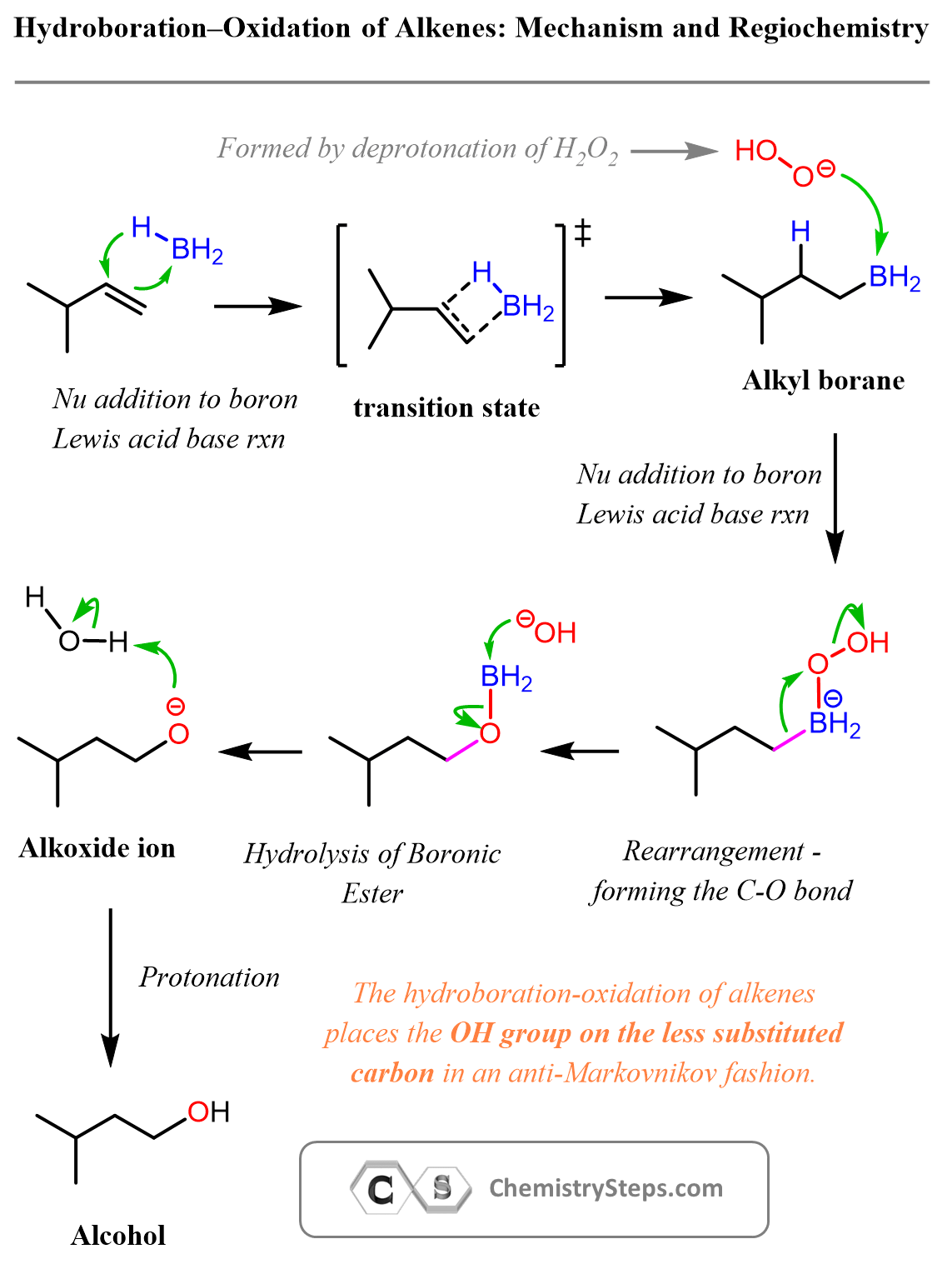
The most important part of this mechanism is the first step. Notice that the BH₃ adds to the less-substituted carbon of the double bond, and this is where the OH of the final alcohol ends up being.
Why am I saying the most important part? They are all important, yes, but the only reason we use BH₃ instead of acid-catalyzed hydration is the anti-Markovnikov regiochemistry, so we need to focus on that key step of the reaction.
Other than the addition of the boron, the other, perhaps confusing step of the mechanism is the 1,2-alkyl shift from the B to the oxygen of the peroxide. It is not as obvious as the other steps, but recall that we have seen rearrangements in SN1 and E1 reactions, and this one is pretty close to what happens in ring-expansion rearrangements, so take some time to refresh those patterns for a better understanding of what is going on in hydroboration-oxidation of alkenes.
The Stereochemistry of Hydroboration Oxidation
So far, we have talked about the regiochemistry and mechanism of hydroboration-oxidation. Another key point to understand is its stereochemistry. Both the boron and the hydrogen add to the same side of the double bond, which means the reaction proceeds with syn addition. As a result, in a cyclic or stereochemically defined alkene, the OH group and the H will end up on the same face of the former double bond.

This becomes especially important when working with cis or trans alkenes, as it determines the relative positions of the substituents in the final alcohol product. For example, in the reactions shown above, a pair of enantiomers is formed, and this is a type of question you will see a lot on your tests.
BH₃ and H₂O₂ in Hydroboration-Oxidation of Alkynes
Similar to how the π electrons of the double bond attack BH₃, alkynes, being even more electron-rich, show identical behavior.
The key difference in the hydroboration-oxidation of alkynes, compared with alkenes, is that once the OH adds to the less-substituted carbon, an intermediate called an enol is formed.

In enols, the OH is connected to the double bond, and this turns out to be not the most stable combination. and they convert by themselves to aldehydes or ketones via a process known as keto-enol tautomerization:

Notice that having an internal alkyne would mean there is no “less-substituted carbon,” thus there would be little to no difference whether we use acid-catalyzed hydration or hydroboration-oxidation. When unsymmetrical internal alkynes are used, a mixture of ketones is formed, no matter what method we use for the hydration of the triple bond.

You may have guessed that symmetrical alkynes can only form one ketone, again, regardless of how they are hydrated.
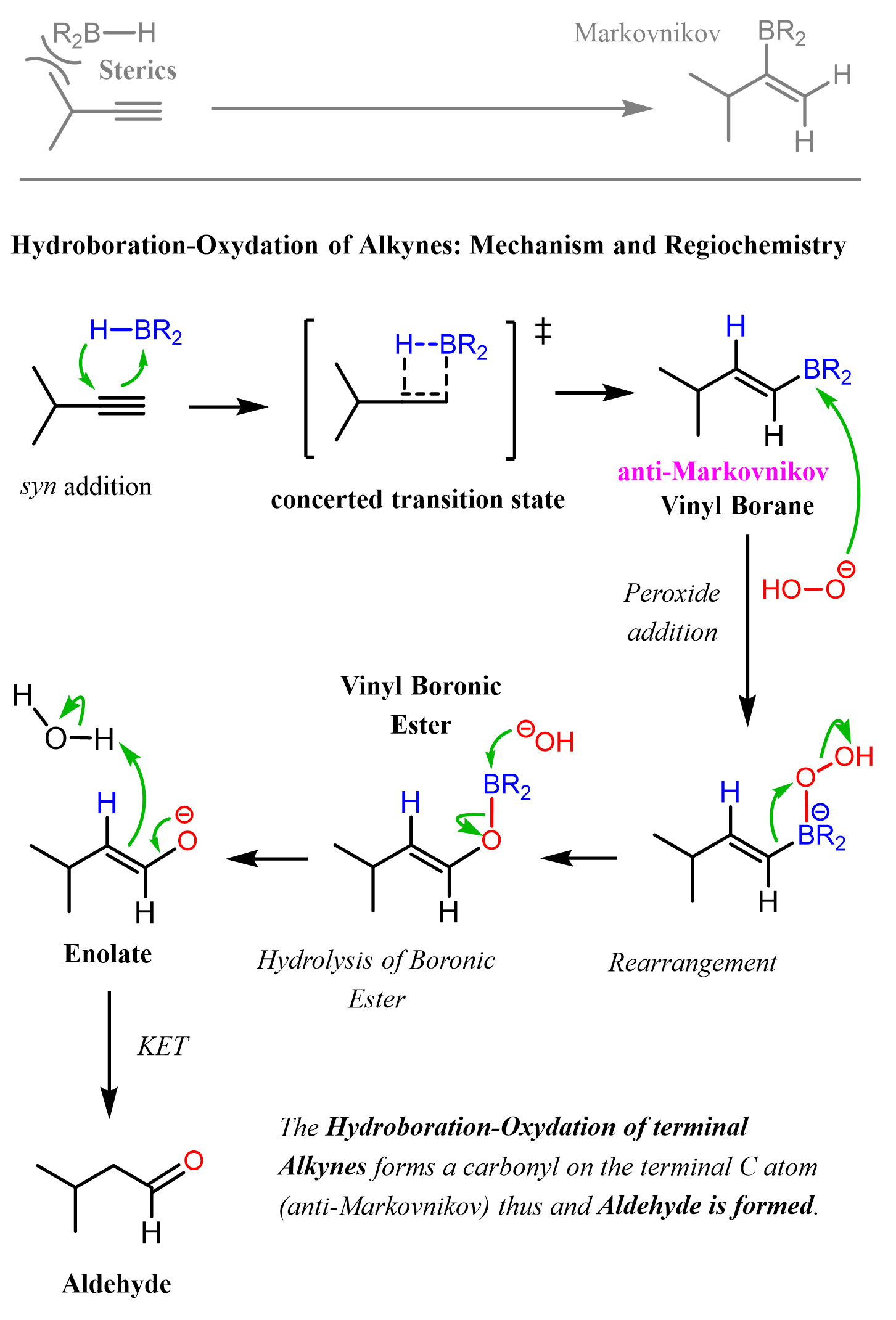
Another difference, which comes from the structures of the alkenes and alkynes, is that for hydroboration-oxidation of alkynes, we use R₂B–H and not BH₃. So, instead of three hydrogens, we have one hydrogen and two alkyl groups on the boron. The reason for this is that alkynes are not only planar but also linear, so there is very little steric hindrance on the side of the triple bond where another carbon is connected. Therefore, the alkyl groups on the alkyl boranes must be very bulky to be effective.
The most common bulky alkyl boranes used for hydroboration-oxidation of alkynes are 9-BBN and Disiamylborane (Sia₂BH):
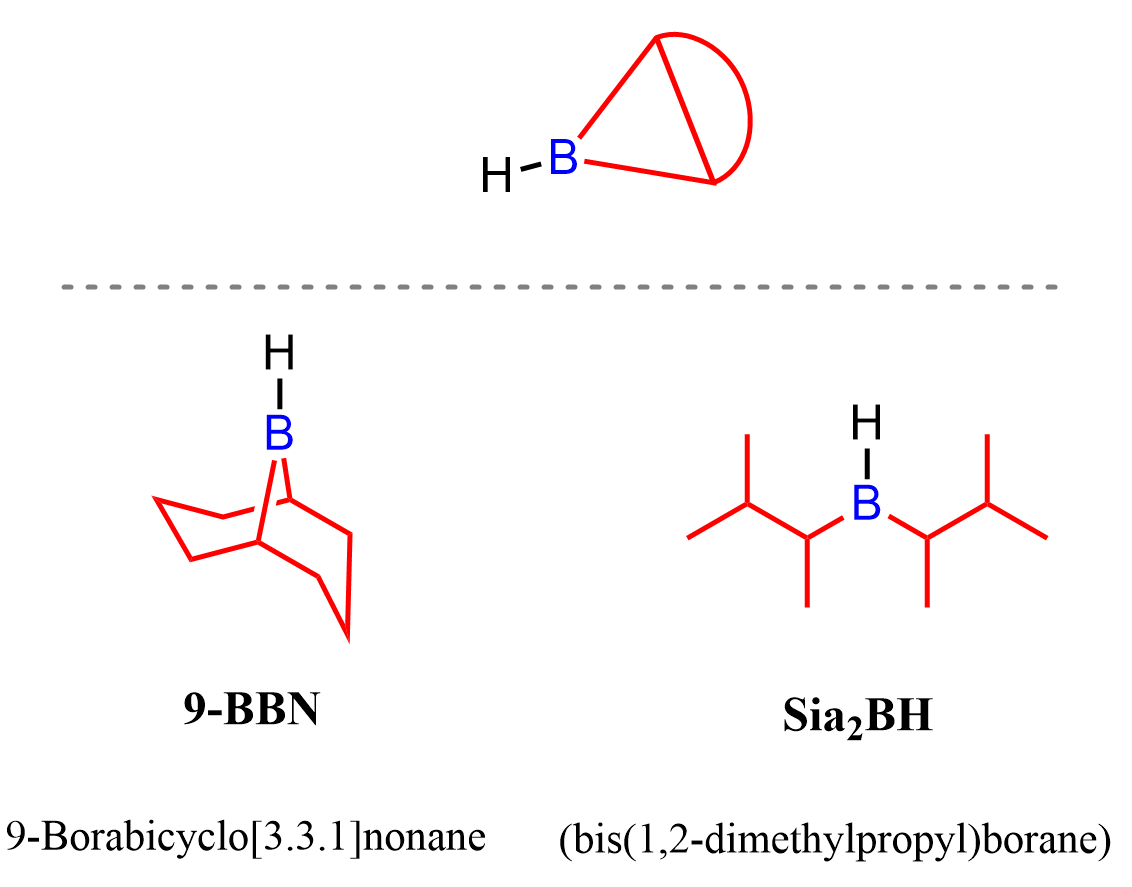
To simplify things and avoid having to remember and draw the bulky substituents, 9-BBN is typically shown as a skeletal sketch rather than its full structure.

Notice that the bulky nature of R₂BH is also helpful in preventing the second addition of the alkyl borane. Alkyl boranes can also be used for the hydroboration-oxidation of alkenes to increase the regioselectivity of the addition to the double bond.
The Mechanism of Hydroboration-Oxidation of Alkynes
Like with alkenes, the reaction starts with a concerted addition of the alkylborane to the triple bond, forming a vinyl borane. Due to the steric hindrance of the bulky alkyl groups, it is the terminal carbon of the triple bond that adds to the boron, forming the anti-Markovnikov vinyl borane intermediate.
In the subsequent steps of oxidation and rearrangement, the BR₂ group is replaced with an OH group, which, after a keto-enol tautomerization, forms the final product, aldehyde:
Summarizing What BH3 with H2O2 Does
Hydroboration-oxidation is a key reaction for adding water across a π bond of alkenes and alkynes with anti-Markovnikov selectivity. The principle is the same; however, the outcome is different.
- Alkenes give alcohols, with the OH ending up on the less substituted carbon.
- Alkynes, on the other hand, first form unstable enol intermediates, which undergo a keto-enol tautomerization to form a carbonyl compound:
- Aldehydes are formed from terminal alkynes.
- Ketones are formed from internal alkynes.
Using bulky boranes like 9-BBN or Sia₂BH ensures regioselectivity and helps prevent overreaction.
Other methods and specifics of hydration reactions of alkenes, such as acid-catalyzed hydration and the use of mercury salts, are covered in the following two articles. Check them out for broader coverage and practice problems on the hydration reactions of alkenes and alkynes.
Check Also
- Markovnikov’s Rule
- Markovnikov’s Rule with Practice Problems
- Addition of Water to Alkenes
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Rearrangements in Alkene Addition Reactions
- Oxymercuration-Demercuration
- Addition of Alcohols to Alkenes
- Hydrohalogenation of Alkynes
- Addition of Water to Alkynes
- Acid-Catalyzed Hydration of Alkynes with Practice Problems
- Alkene Addition Reactions Practice Quiz
- Reactions Map of Alkenes
Alkyne Naming and Reactions Practice Quiz
Reactions Map of Alkynes
Other reactions of Alkenes and Alkynes can be found on the Organic 1 and 2 Topics page.
