We have seen in many reactions that amines are quite good nucleophiles and strong bases, and therefore, sometimes, they need to be protected to allow for transformations of other functional groups.
Perhaps the most common strategy to protect an amino group is to convert it into a carbamate. Don’t get stressed over the new name carbamate as it is simply an ester of the carbamic acid, just like it would acetate for the esters of acetic acid:
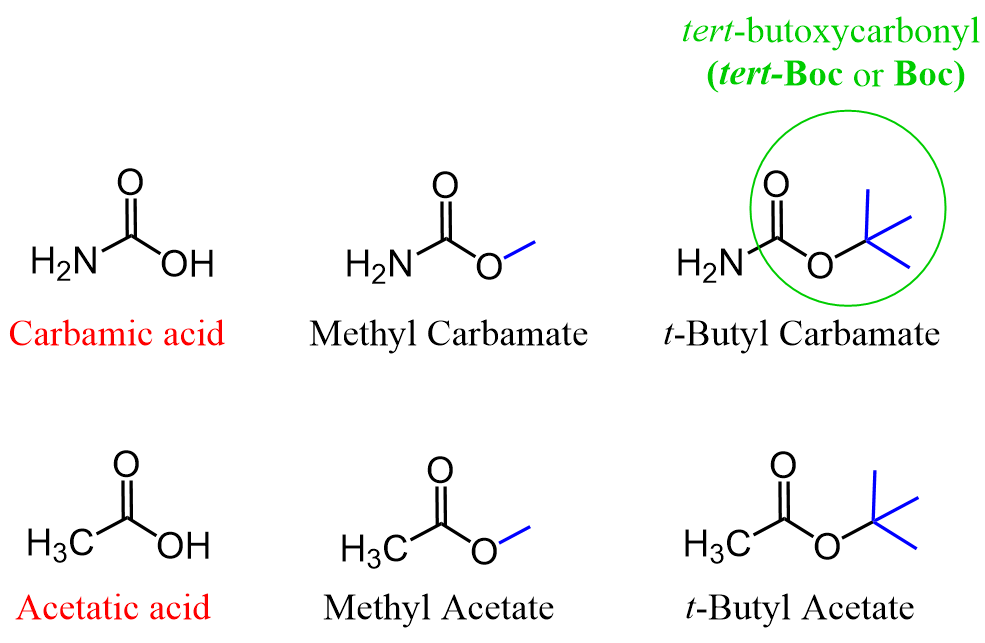
So, let’s understand why the tert-butyloxycarbonyl (t-Boc or simply Boc) is a better option than, for example, a methoxycarbonyl derivative. This has to do with the stability of the more substituted tert-butyl carbocation which is cleaved when the Boc protecting group is removed.

Now, since we talked about it, let’s go ahead and show the mechanism of cleaving the Boc group which is achieved with a strong acid such as trifluoracetic acid (TFA).
The first step would be protonation of the carbonyl oxygen since the resulting ion is resonance stabilized.

We have discussed why this oxygen acts as a Lewis base in the article about the reaction of SOCl2 with carboxylic acids. The resonance stabilization puts a partial positive charge on the oxygen bonded to the t-butyl group, which facilitates its cleavage:

The resulting carbocation can be stabilized by undergoing an elimination by the trifluoroacetate ion. Notice that having a less substituted alkyl group would make the cleavage of the alkyl group significantly more difficult and that is why the tert-butyloxycarbonyl (Boc) is used, and, in fact, this reaction is typically very easy, and we can see it happening by the CO2 bubbling out of the solution.
Where is the CO2 coming? Well, that is the decarboxylation which, we can say is the last part of the deprotection.
Ok, now that we know why Boc is a good choice to form a carbamate protecting group, let’s also see how it is added to the amine. The precursor is Di-tert-butyl decarbonate which is a great electrophile for a nucleophilic addition of the amine:

The first part is a typical nucleophilic addition-elimination, which we have seen many times in the reactions of carboxylic acid derivatives, forming a tetrahedral intermediate. In the elimination step, a carbonate ion is expelled, and it can either deprotonate the amine or undergo a spontaneous decarboxylation yielding a strong base tert-butoxide to take care of the amine:

Let’s also combine the addition and the removal of the Boc group to summarize the mechanisms:

The Boc group is the most used protection of amino groups for example in the synthesis of peptides, but let’s also discuss the phenylmethoxycarbonyl group (abbreviated carbobenzoxy or Cbz).

The benzyl group is usually stable under acidic and basic conditions and is cleaved by catalytic hydrogenation with H2 over Pd/C. And because of this, it is not suitable for many reactions that involve a double or a triple bond.
Check Also
- Naming Amines: Systematic and Common Nomenclature
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
- The Cope elimination
- Basicity of Amines
