Carbohydrates, also known as sugars and starches, are one of the most important classes of compounds found in nature.
Listing only a few examples would include the energy produced in our bodies by breaking down sugars which are used in most biochemical processes. And by sugar, we are not only talking about the table sugar and other sweet things but anything containing carbohydrates such as fruits, starchy vegetables, grains, bread, corn, rice, etc.

Unlike us, plants synthesize carbohydrates and create this storehouse of chemical energy through photosynthesis which works by absorbing the sunlight and using it to facilitate energy and electron transfer processes. In an extremely simplified summary, it is a reaction between water and carbon dioxide eventually resulting in the formation of carbohydrates.
Carbohydrates, along with nucleobases are the building components of nucleotides which build the genetic materials DNA and RNA:

Starch and cellulose are very similar structurally. However, while starch is found in large amounts in wheat, potatoes, corn, and rice, cellulose is the most abundant organic polymer on Earth. It makes the stems and tree trunks, a large content in cotton fiber (90%), wood (40–50%), and dried hemp (60%). It is also responsible for the rigidity of different animal shells.

So, what makes starch and cellulose so different?
Glucose, the building unit of starch and cellulose, is a six-membered ring that adopts a chair conformation and as a result, it has axial and equatorial groups. The only difference when making the polymer is that the oxygen, in starch, linking the units uses one equatorial and one axial bond while in cellulose it links them by two equatorial bonds.

The human body contains an enzyme capable of hydrolyzing the axial bond in starch and making glucose while it leaves the equatorial bonds intact hence, we cannot digest cellulose.
The abundance and importance of carbohydrates is incredibly interesting. However, the focus of this and the following posts is the structure, classification, and the chemistry rather than their role and mechanism in biological processes. These are covered in biochemistry and to understand the biochemistry of sugars and other compounds, we first need to have the basic structural foundation.
The Structure and Classification of Carbohydrates
Let’s start with the name – why do we call them carbohydrates?
The reason for this is simply because they have a general formula of Cn(H2O)n, so mathematically it is one carbon per one water molecule – a carbohydrate. For example, when n=6, we have 6 x C with 6 x H2O = a formula of C6H12O6 which is the well-known Glucose. Keep in mind though that this is only a mathematical match – the structure, elucidated later after their discovery, and the properties of carbohydrates are not related to that of water and carbon.
Each individual sugar molecule is referred to as monosaccharides which are the simplest carbohydrates or simple sugars often shown in Fischer projections. A lot of carbohydrates, including glucose, are composites of many carbohydrate molecules which classifies them as di-, oligo- or polysaccharides.
Monosaccharides have three to seven carbon atoms and which is specified with a prefix “tri”, “tetra”, “pent” etc. They are all named by combining the prefix with the common suffix “ose”.

If it’s been a while, you can read this article about the Fischer projections for more details. In short, you recall that the horizontal groups are pointing towards you and the ones on the vertical line are pointing away from you.
For example, looking at glyceraldehyde, which is the simplest carbohydrate, we need to understand that the H and OH groups are pointing towards us, while the aldehyde and alcohol are pointing away from us:
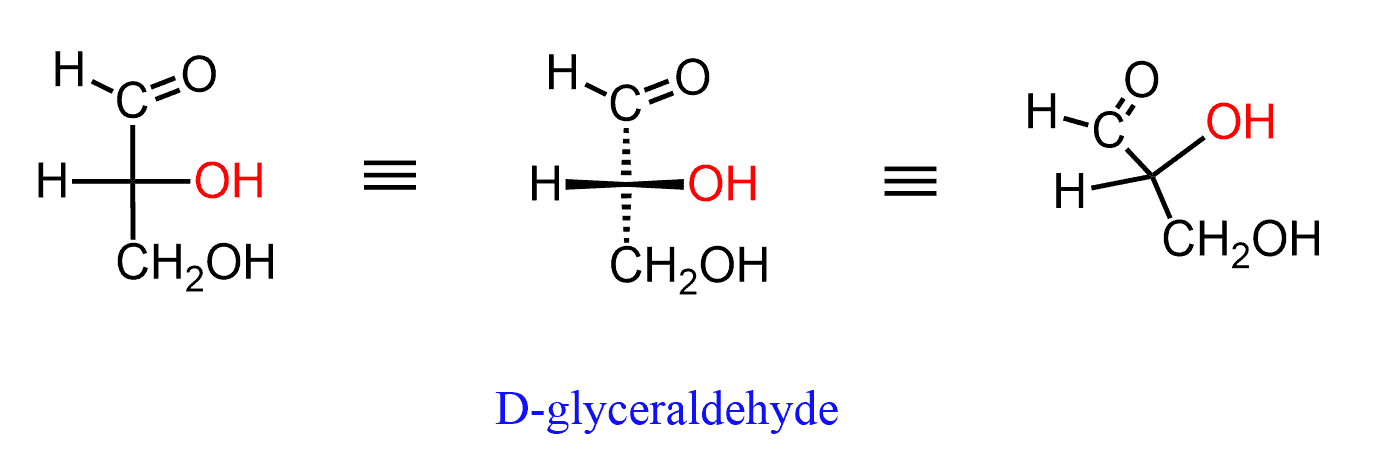
Remember also that Fischer projections cannot be rotated by 90o. The 180o flip is allowed and the reason for this is the fact that the absolute configuration of the chirality centers must be retained since it is the same molecule. This is only possible if the horizontal groups stay as horizontal and vertical groups stay as vertical as well:

If you rotate the molecule by 90o, the horizontal groups get in the vertical positions, which in Fischer projections means that they are now pointing away from you. This changes the absolute configuration from S to R and therefore you are showing the enantiomer of the molecule:

Aldose or a Ketose Notation
Depending on the position of the carbonyl (C1 or C2) the sugar molecule can be an aldehyde or a ketone which are classified as an Aldose or a Ketose:

You might have noticed the indefinite articles in front of the names and wondering why we need them. This has to do with the stereochemistry of carbohydrates. Each chiral center can be inverted and thus a pair of enantiomers or diastereomers are possible for the given sugar. The stereochemistry is indicated by the old, and yet common L and D notation and the traditional R and S configuration.
We will cover this in the following post: Stereochemistry of Carbohydrates
Cyclic Structures of Carbohydrates
Remember, earlier in this post, we mentioned that glucose is a six-membered ring that adopts a chair conformation and as a result, it has axial and equatorial groups? Yes, even though so far, most structures we have seen have been shown in Fischer projections, carbohydrates exist also in a cyclic form.
For example:

Notice that the cyclic forms of ribose and glucose are labeled as a furanose and a pyranose. These are general names for 5 and 6-membered rings containing an oxygen atom:

- Pyranose – a six-membered ring containing an oxygen
- Furanose – a five-membered ring containing an oxygen
Furanose and pyranose rings are both hemiacetals and to understand the formation of the cyclic carbohydrates, we need to remember the reaction between aldehydes and ketones with alcohols which forms acetals:
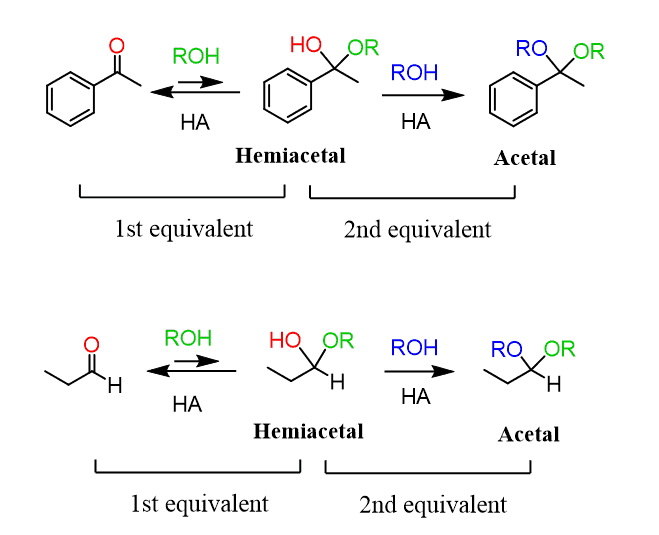
If the starting material contains both a carbonyl and an OH group, an intermolecular reaction occurs forming a cyclic hemiacetal:
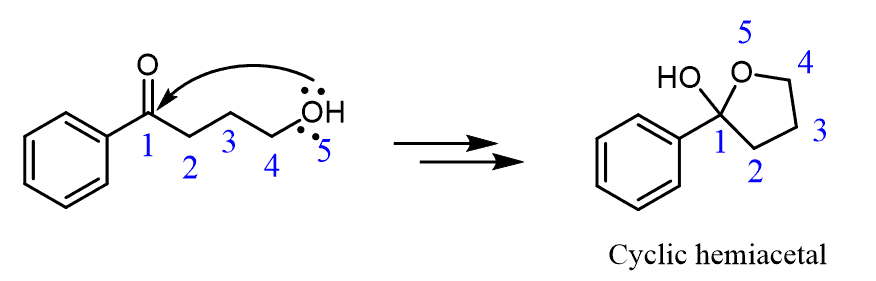
This is what we have in the formation of furanose and pyranose rings. Remember, that carbohydrates contain a carbonyl and an OH group, and this how they interconvert between the linear and cyclic forms:

As strange as it may look, the hemiacetal on the right represents glucose in a cyclic form!

Again, this may look off, and converting carbohydrates from a linear to a cyclic form might be a little tricky as, besides the sudden change of the entire shape, we need to understand the stereochemistry and make sure to keep it consistent across all the chiral centers. This deserves a separate post and we will surely do so.
Summary of Carbohydrate Introduction
Let’s summarize what we learned about carbohydrates so far.
- Carbohydrates are extremely abundant and important compounds.
Why do we call them carbohydrates?
- They have a general formula of Cn(H2O)n, so mathematically it is one carbon per one water For example, n=6 gives C6H12O6 – Glucose.
- What are carbohydrates in chemical terms?
Carbohydrates are aldehydes or ketones with multiple hydroxyl groups. They are also known as sugars.
- A single carbohydrate unit is called a monosaccharide. These contain from 3-7 carbon atoms often shown in Fischer projections. Carbon 1 or 2 is a carbonyl and the rest are OH groups.
- Based on the number of carbon atoms, we have the following general name: 3 carbons-Triose, 4 carbons-Tetrose, 5 carbons-Pentose, 6 carbons-Hexose.
- Depending on the position of the carbonyl (C1 or C2) it can be an Aldose or a Ketose:

- Carbohydrates exist also in a cyclic form – Pyranose (6-membered) and Furanose (5-membered) rings:

Hopefully, this post is a good introduction to carbohydrates and gives you some relief from the overwhelming looks and names of sugars you encountered before. We will cover the details of each paragraph in the upcoming posts.
Need some practice on carbohydrates?
Check this Multiple-Choice, summary quiz on the structure and reactions of carbohydrates with a 40-min video solution!
Carbohydrates Practice Problem Quiz
Check also in Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation

