We discussed quite extensively the aldol reactions of aldehydes and ketones which involve enolate ions generated by base-catalysis. Esters are known to undergo an analog reaction called Claisen Condensation since they too have an acidic ɑ position to form an enolate and, of course, a carbonyl to serve an electrophile.
Let’s put an aldol and a Claisen reaction next to each other to compare and see the analogy:

So, just like in the aldol reaction, one molecule forms an enolate and attacks as the carbonyl group of the other ester. The product in the Claisen condensation is a β-keto ester, and for the aldol addition, it is a β-hydroxy carbonyl. The main difference in the products is that, in Claisen reaction, both molecules retain their carbonyl group, and this is due to the presence of the alkoxy group which serves as a leaving group after the nucleophilic addition:

Notice that the reaction is an equilibrium and all the steps are reversible except the very last one. To start with, the ethoxide is not a strong enough base to fully deprotonate the ester, so the enolate is formed in very low concentration. However, being a strong base, it reacts with another molecule as soon as it is formed.
The next step is also unfavorable, because β-keto esters are less stable than the esters they are derived from.
With all this said, there must be a driving force to push this reaction forward and that is the deprotonation of the final product.
Now, remember that dicarbonyl compounds are very acidic – about twice more acidic as their analogs with one electron-withdrawing group:

Therefore, the β-keto ester formed in the last (colored) step is deprotonated by the ethoxide still present in the reaction mixture. This deprotonation is an irreversible acid-base reaction and is the driving force that shifts the equilibrium in favor of the condensation.
This, in turn, means that if the ester had only one ɑ hydrogen, the reaction wouldn’t work since it is removed in the first step and there will be no more to be removed and shift the equilibrium forward. So, it’s important to keep in mind that Claisen condensation only works for esters with two or three ɑ hydrogens:
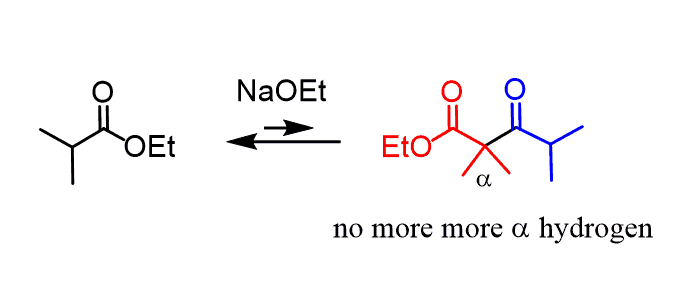
The second part of the reaction is an acidic workup to make sure a neutral product is obtained.
The Choice of Base in Claisen Condensation
For a given Claisen reaction, there is essentially one suitable base that can be used. And that is the alkoxide ion having the same alkyl group as the reacting esters.
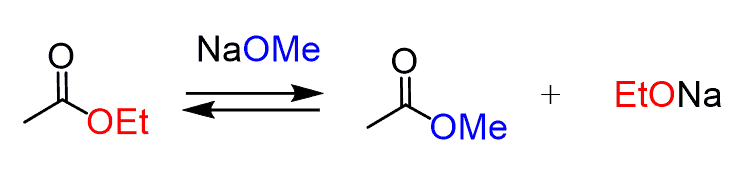
For example, to deprotonate the ethyl acetate or any other ethyl ester, an ethoxide salt must be used. If a methoxide base is used, a problem of transesterification occurs:
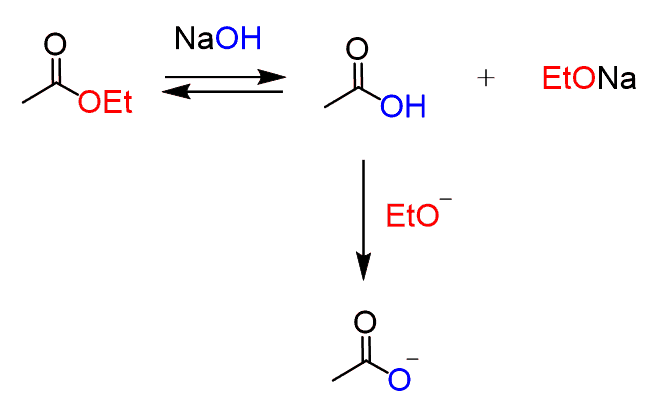
A hydroxide base, on the other hand, may hydrolyze the ester to a carboxylic salt:
So, besides being restricted to use the specific alkoxide base for a given Claisen reaction, another option is to use a strong-non nucleophilic base such as LDA to prevent the issue of transesterification. LDA is used for the crossed aldol reaction but in general, it is not the base choice if you can get away with using a weaker base and carrying the reaction in milder condition.
You can find more examples and practice problems on the Claisen condensation on th elink below:
https://www.chemistrysteps.com/claisen-condensation-practice-problems/
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem

I need the reaction mechanism steps for this reaction :
Claisen condensation of acetone and ethyl formate to form 1,3,5-TRIACETYLBENZENE
Thank you very much for the information.
If you have tutorial videos on YouTube kindly share the link.
Thank you in anticipation.