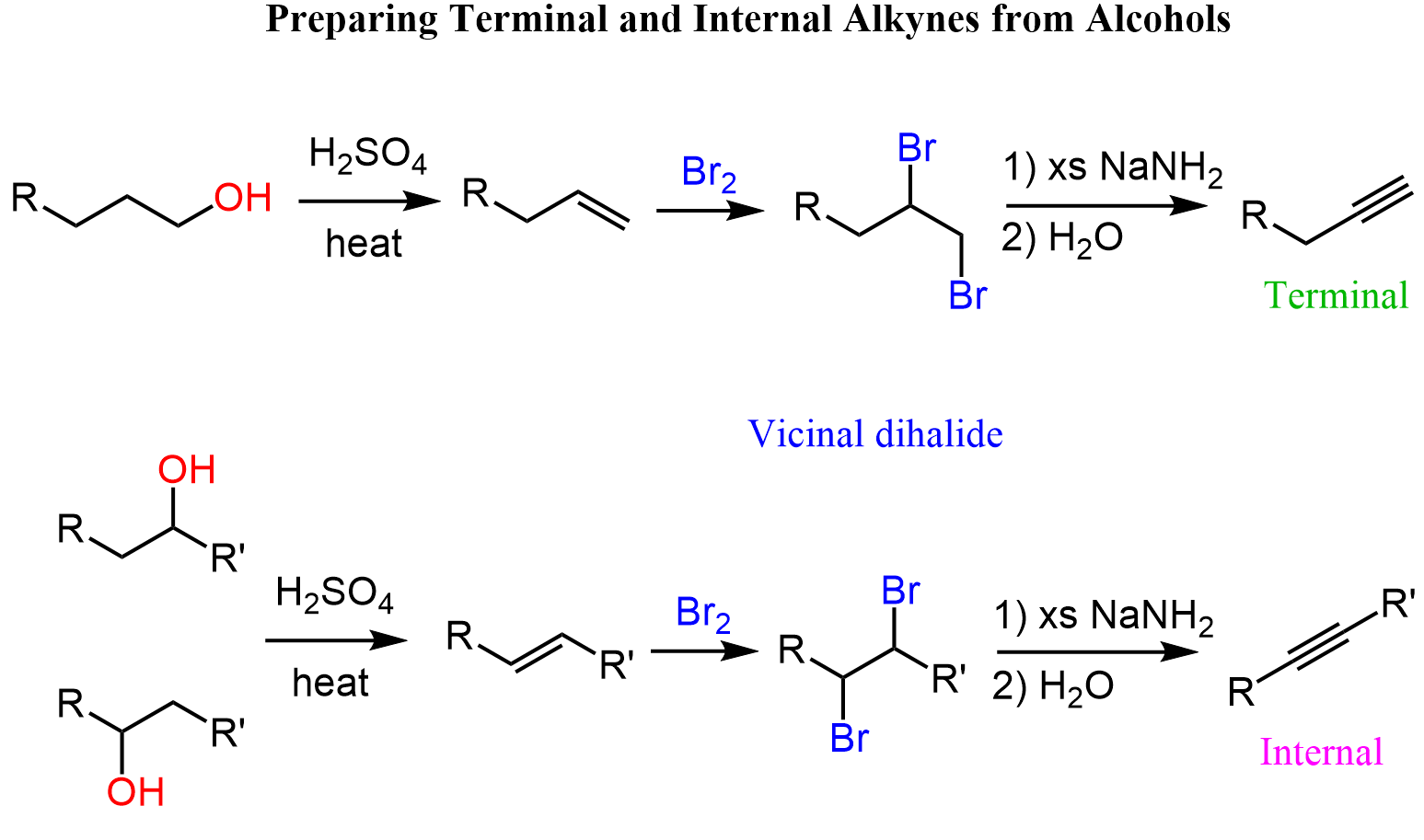
The conversion of alcohols to alkynes is a two-step process. We need to first dehydrate to an alkene, and halogenate the latter to a dihalide, which can now be treated with a strong base such as sodium amide (NaNH2) to achieve a double elimination:
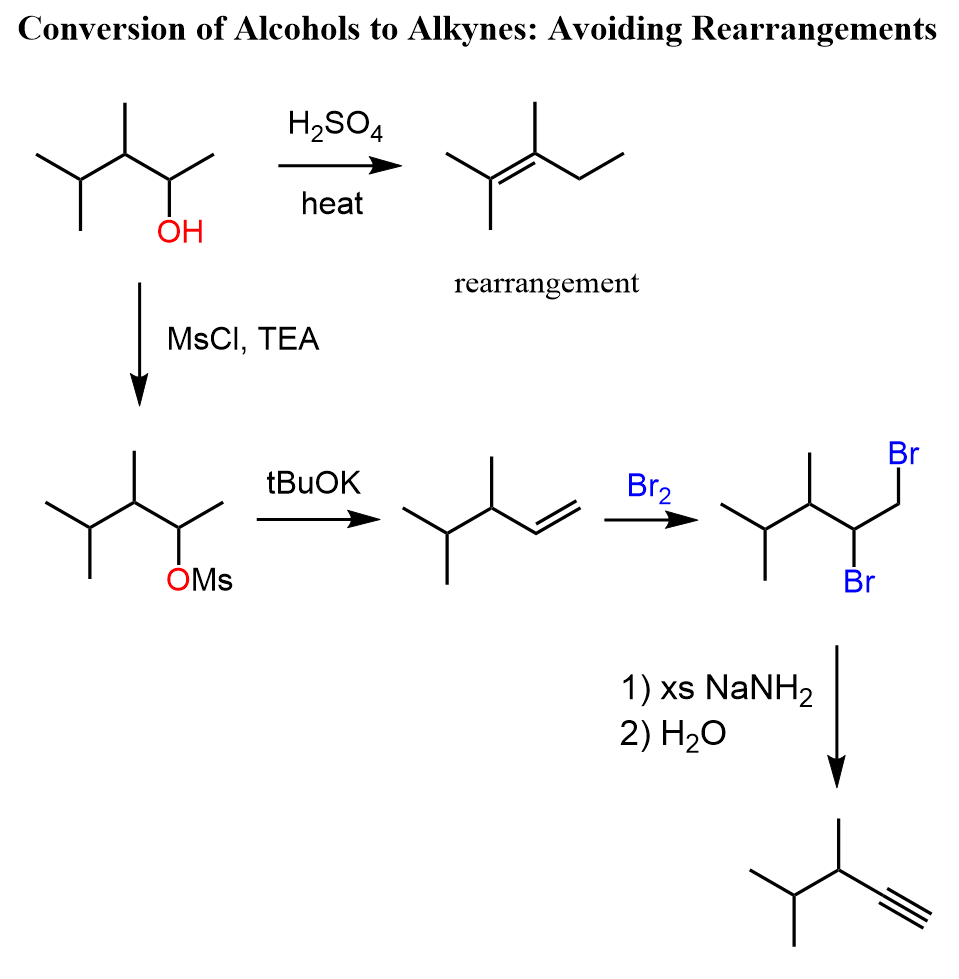
Remember that the acid-catalyzed dehydration of alcohols goes via carbocation intermediate thus rearrangements are possible. To avoid rearrangements, you can convert the alcohol to an alkyl halide, mesylate, or a tosylate and do a Zaitsev or Hofmann elimination to prepare the needed alkene:
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Introduction to Alkynes
- Naming Alkynes by IUPAC Nomenclature Rules – Practice Problems
- Preparation of Alkynes by Elimination Reactions
- Hydrohalogenation of Alkynes
- Addition of Water to Alkynes
- Acid-Catalyzed Hydration of Alkynes with Practice Problems
- Reduction of Alkynes
- Halogenation of Alkynes
- Hydroboration-Oxidation of Alkynes with Practice Problems
- Ozonolysis of Alkynes with Practice Problems
- Alkylation of Terminal Alkynes in Organic Synthesis with Practice Problems
- Alkyne reactions summary practice problems
- Alkyne Synthesis Reactions Practice Problems
- Alkyne Naming and Reactions Practice Quiz

