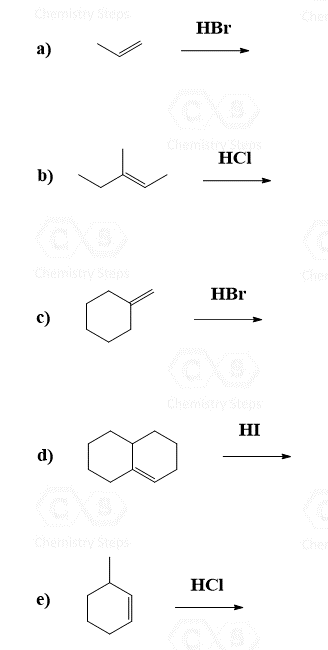
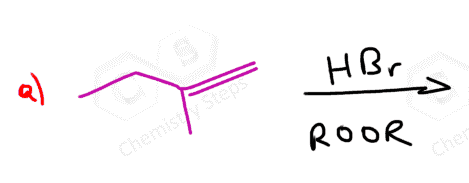
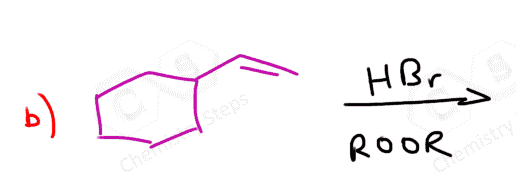
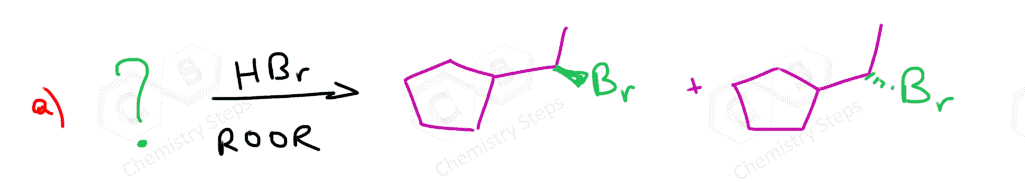
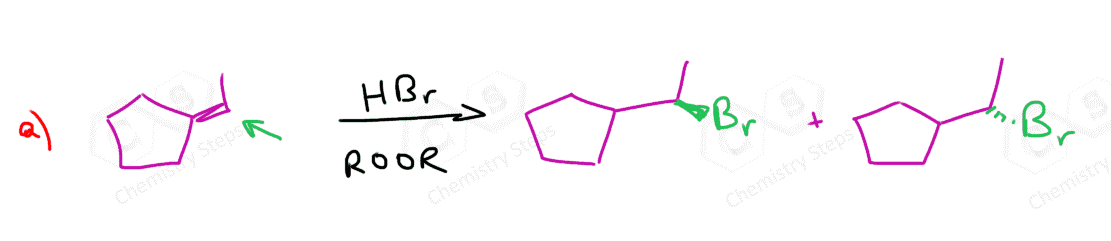
Alkenes are converted to alkyl halides by the addition reactions of hydrohalic acids (HCl, HBr, HI). These are generally Markovnikov additions unless we direct the Br to the less substituted carbon atom radical addition using a peroxide catalyst:

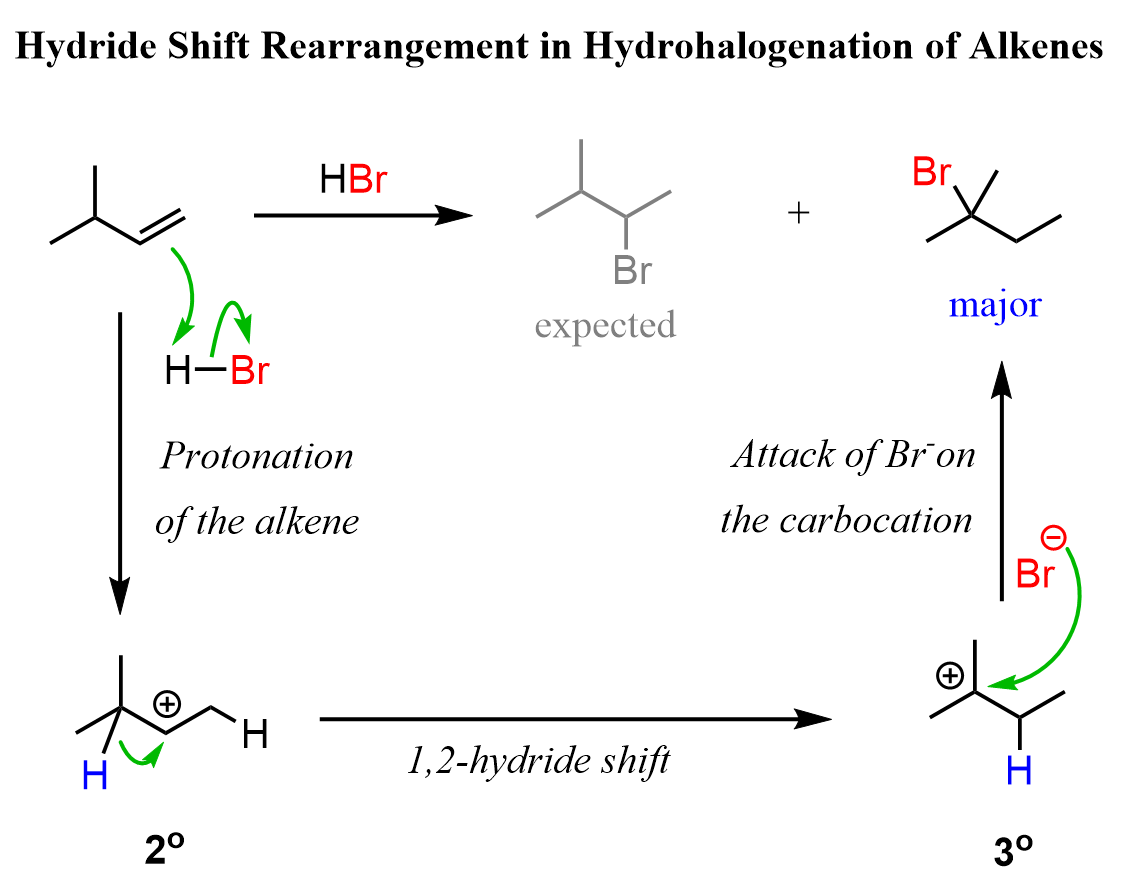
From reaction (3), we can see that one problem in converting alkenes to alkyl halides is the possible rearrangement of the carbocation intermediate.
So, let’s see how we can convert 3-methylbut-1-ene to 2-bromo-3-methylbutane. The major product of this reaction is 2-bromo-2-methylbutane because of a 1,2-hydride shift rearrangement:
So, how can we achieve this transformation?

We can first convert the alkene to the corresponding alcohol and then substitute the OH with Cl, can’t we?

The acid-catalyzed hydration of alkenes is also a unimolecular reaction with a carbocation intermediate, so the same problem of rearrangement will occur. Even if the needed alcohol was prepared by acid-catalyzed hydration, there was still going to be a rearrangement as the reaction of secondary alcohols with HX acids goes by both SN2 and SN1 reactions.

One way of avoiding rearrangements in addition reactions of water to alkenes is the use of oxymercuration-demercuration. This does a Markovnikov hydration with no risk of a rearrangement, so we can first convert the alkene to a secondary alcohol via oxymercuration-demercuration and convert the alcohol to the corresponding chloride.

As mentioned earlier, we do not simply react the alcohol with HCl because of a rearrangement. We can either convert the alcohol to a mesylate or a tosylate and react it with NaCl in a polar aprotic solvent to enforce the SN2 mechanism, thus avoiding the rearrangement.
Another strategy for converting secondary alcohols to alkyl halides by the SN2 mechanism is the use of SOCl2 or PBr3 reagents.
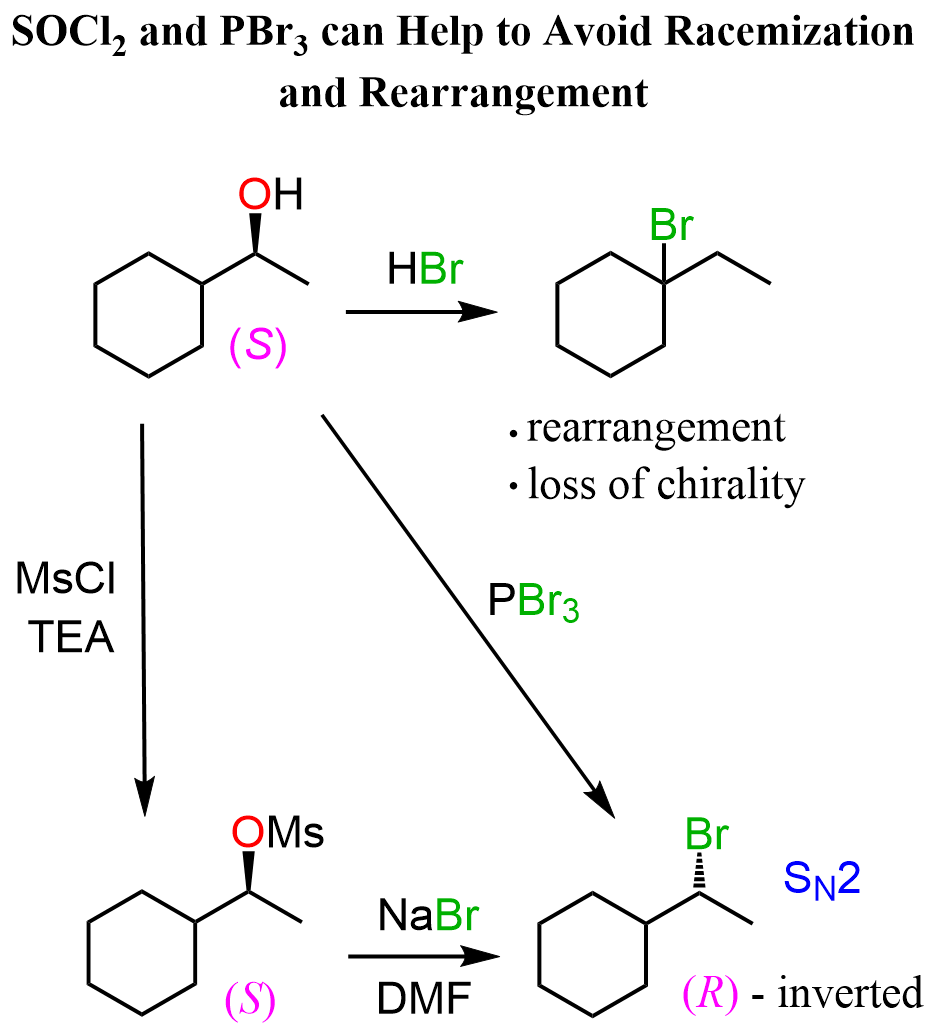
Check this article for more information on converting alcohols to alkyl halides.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!