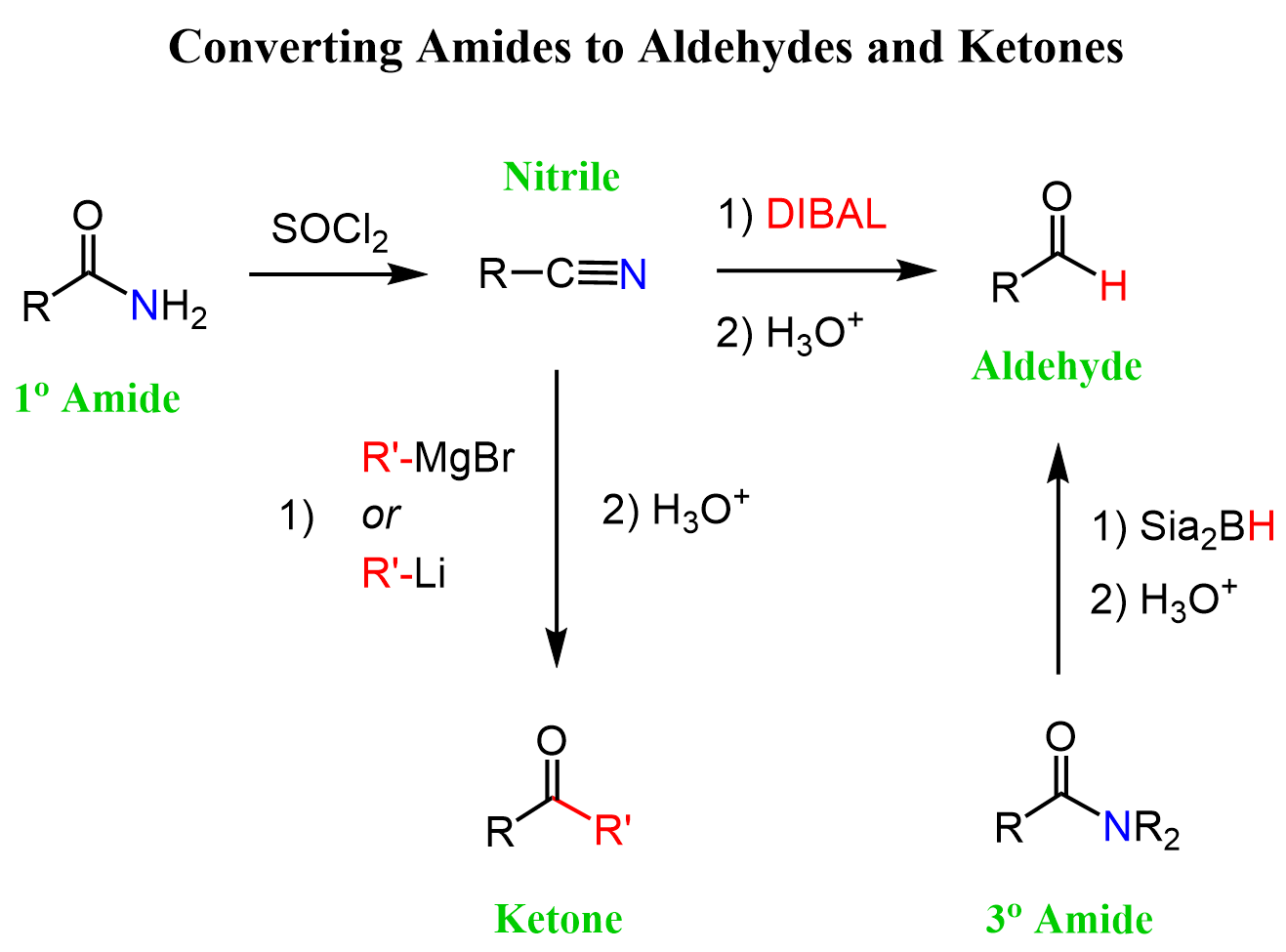
Primary amides can be converted to aldehydes and ketones via nitriles. So, the first thing we need here is a strong dehydrating agent such as SOCl2, P2O5, and POCl3 to convert the amide to a nitrile:

Amides to Aldehydes
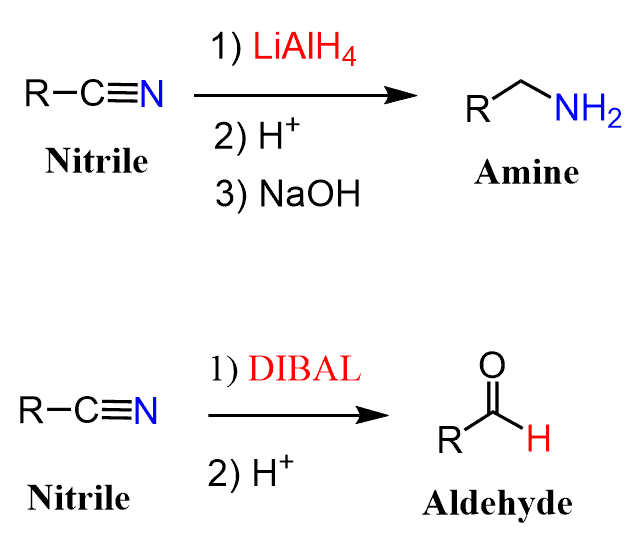
Nitriles can be reduced to aldehydes using the bulky reducing agent DIBAL. The bulkiness of the alkyl groups does not allow for the second hydride addition, and we can quench the reaction by hydrolyzing the intermediate iminium salt to an aldehyde.

More details about the hydrolysis of imines and enamines are covered here.
Notice that using a strong reducing agent such as LiAlH4 reduces nitriles to primary amines:
Direct Conversion of Amides to Aldehydes
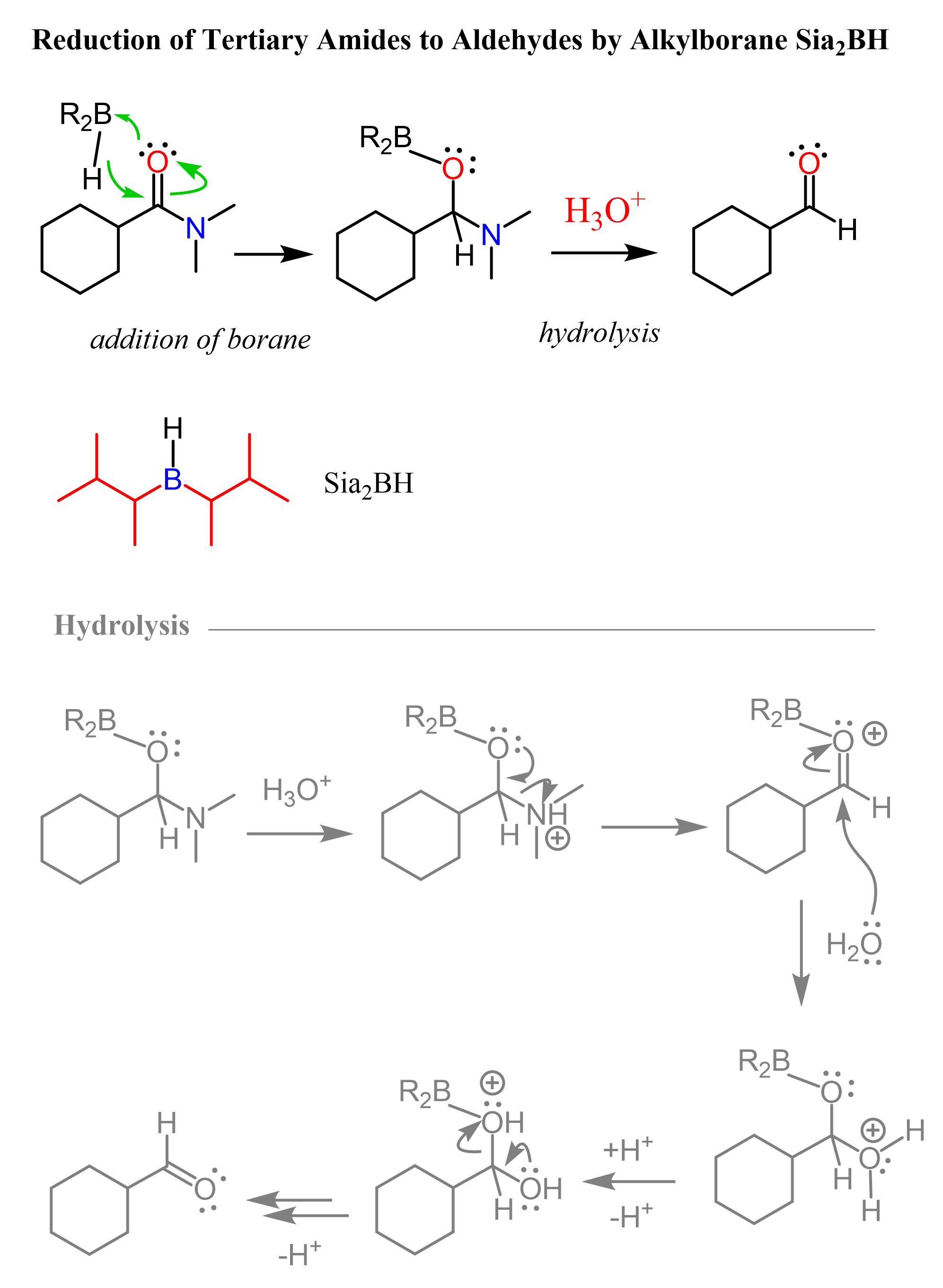
Tertiary amides can be reduced directly to aldehydes using the alkyl borane Sia2BH (Disiamylborane (bis(1,2-dimethylpropyl)borane)). Like in the reduction of nitrile to aldehydes, the second hydride addition does not occur and the hydrolysis of the boronic ester intermediate gives the corresponding aldehyde as the final product:
It is worth mentioning that another alkylborane 9-BBN reduces tertiary amides to primary amines:
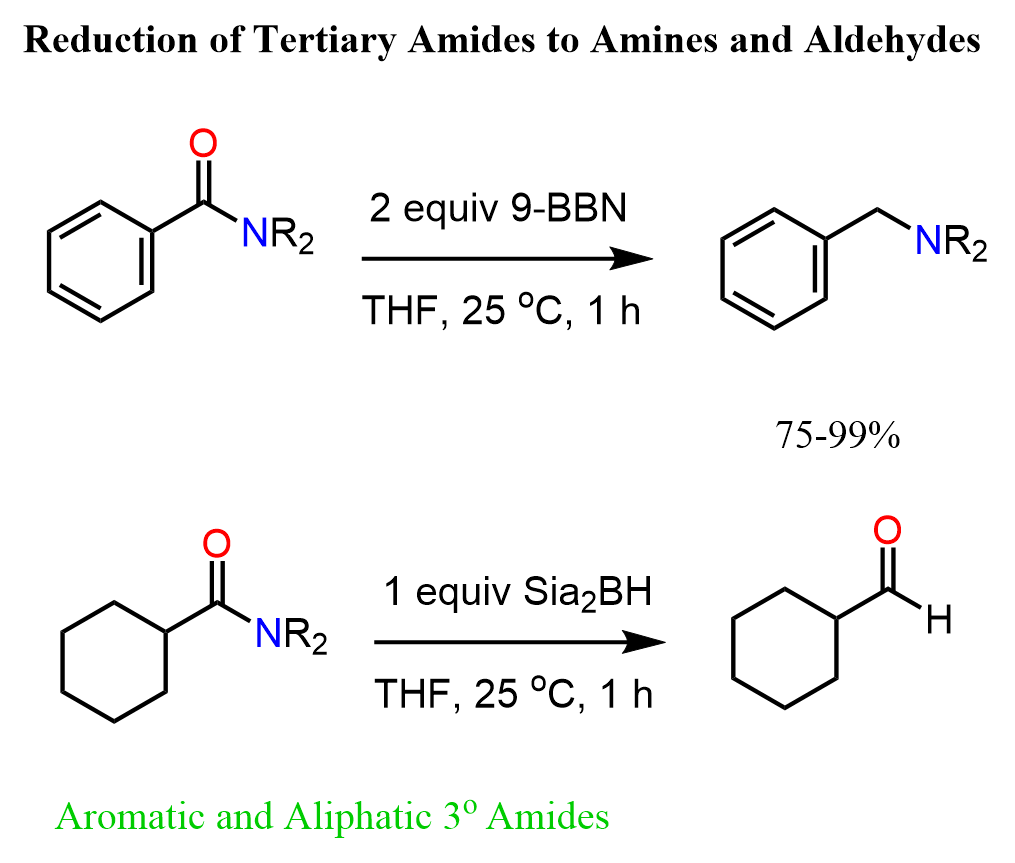
The reduction with alkylboranes is challenging for primary secondary amides because of the formation of C-O and C-N boronic esters.
Here is the original article for further reading: Tetrahedron Letters, Vol. 38, No. 10, pp. 1717-1720, 1997.
Converting Amides to Ketones
To convert an amide to a ketone, we are still going to use an interesting feature of nitriles which is forming ketones when reacted with Grignard and Organolithium agents:

Once again, the intermediate in these reactions is an iminium salt which is hydrolyzed to the corresponding ketone. You can check this article for more details.
Let’s summarize all the strategies we discussed for converting amides to aldehydes and ketones in one synthetic scheme:
Direct Conversion of Weinreb Amides to Ketones
This may be outside your organic chemistry coursework, but it is not something you’d never imagine happening. For example, the Weinreb Ketone Synthesis is a well-known reaction for converting Weinreb (N-methoxy-N-methyl amides) amides to ketones.
Weinreb amide which are most often prepared by a nucleophilic acyl substitution of acid chloride.
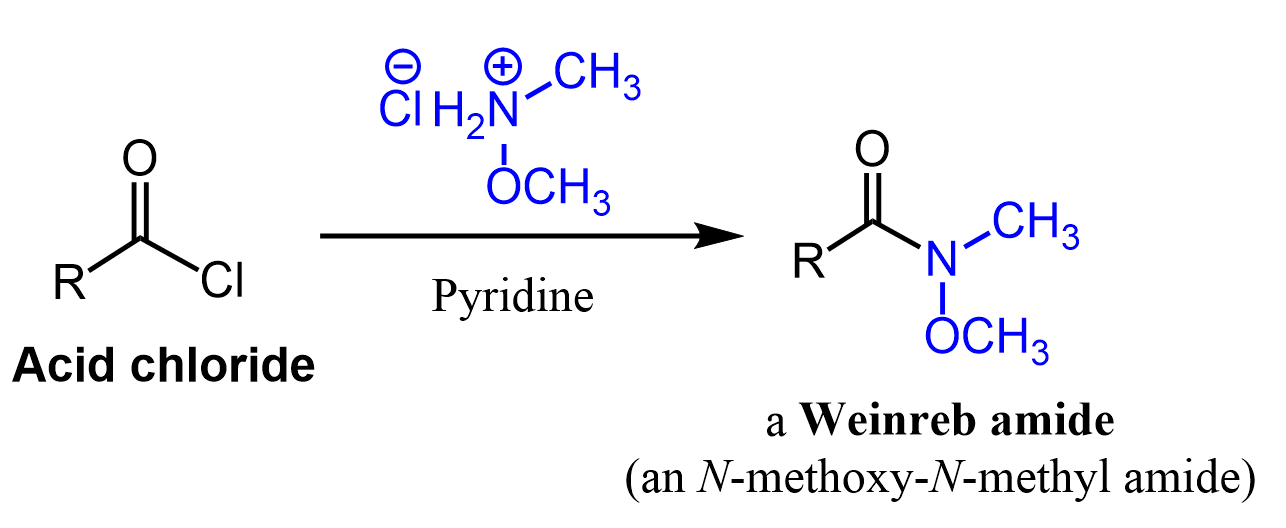
The first step is the nucleophilic addition of the organolithium or organomagnesium to the amide. The tetrahedral intermediate, formed after the addition of organolithium or organ magnesium (Grignard) reagents to the Weinreb amide, is stabilized by chelation of the magnesium atom by the two oxygen atoms.

Weinreb amide is quite stable and can be purified and isolated which makes it a great precursor for different reactions.
Direct Conversion of Regular Amides to Ketones
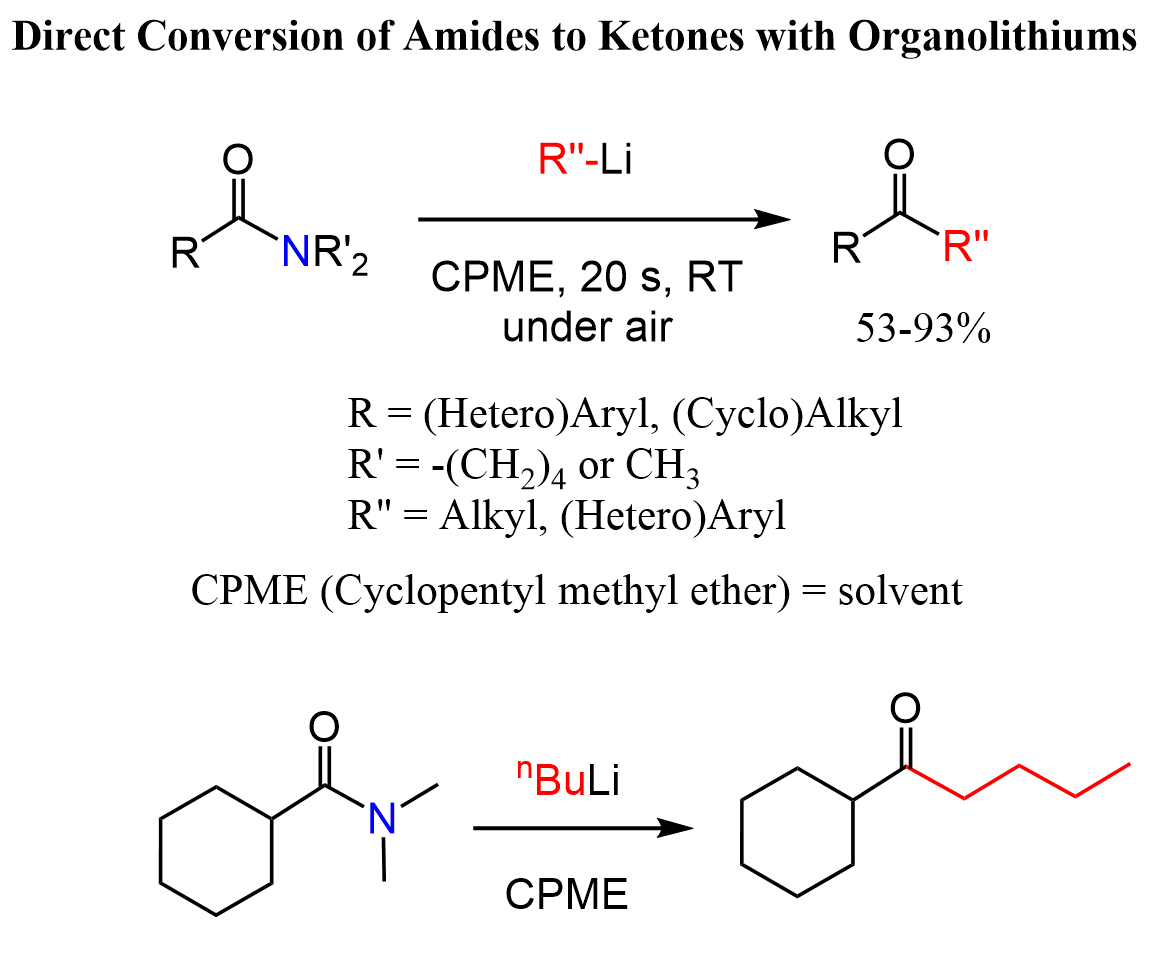
There is recent literature data (for example, https://doi.org/10.1002/chem.202004840) showing that organolithiums can be directly involved in nucleophilic acyl substitution with regular amides converting them to ketones.
Another interesting approach was reported recently where N–Boc amides are converted to ketones with Grignard reagents.
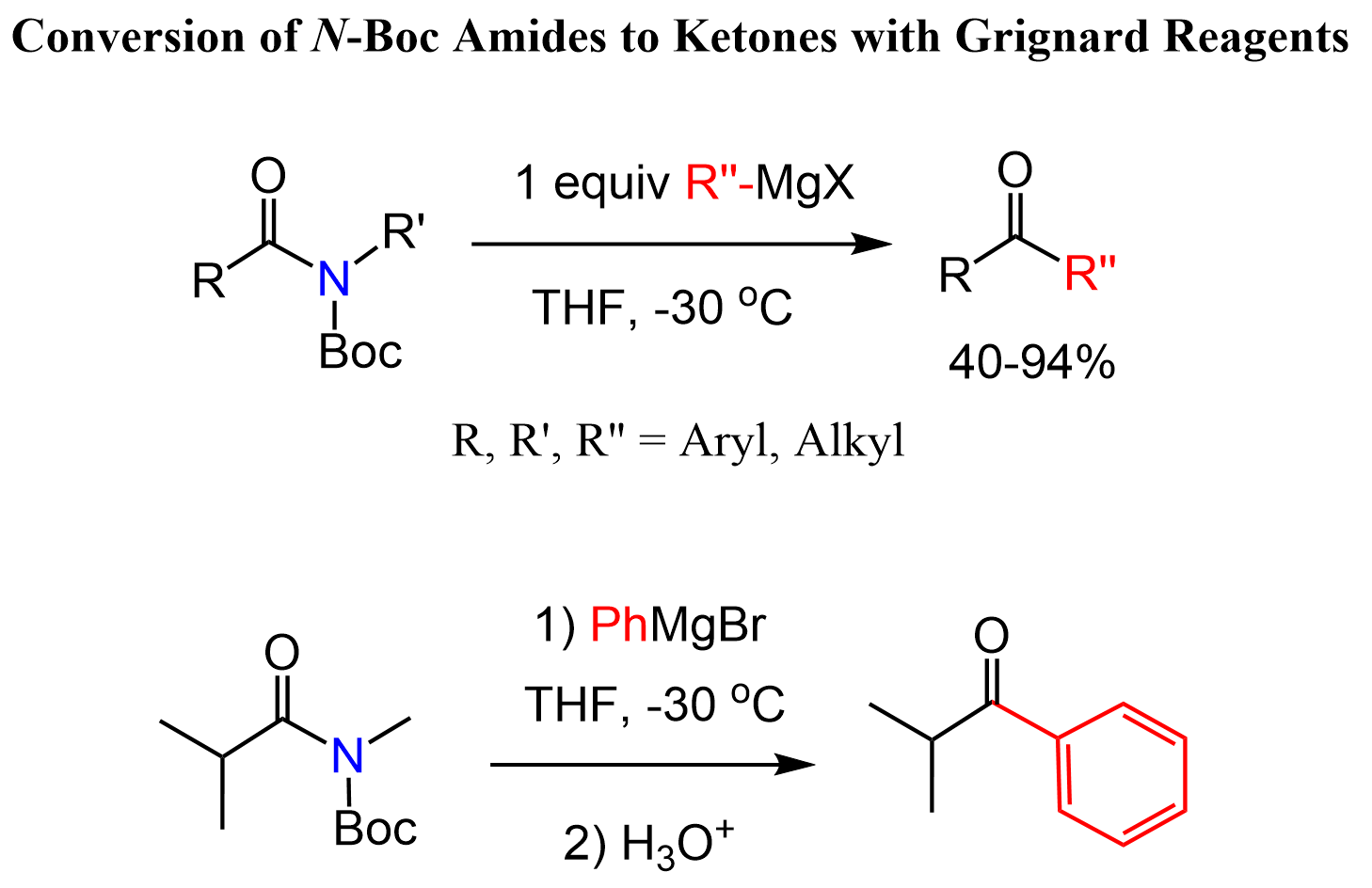
It is also worth mentioning that the authors reported higher reactivity of N-Boc amides towards Grignard reagents compared to Weinreb amides. J. Org. Chem. 2019, 84, 18, 11823–11838
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz

