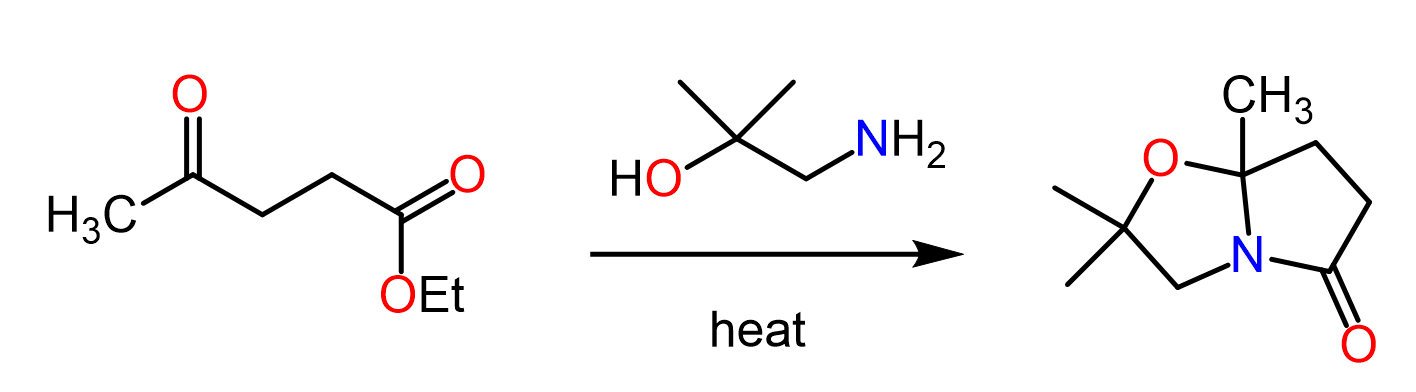
Let’s look at this conversion through the eyes of a student new to organic chemistry: amides are almost like amines, except they contain a carbonyl (C=O) group attached to the nitrogen.
So, how can we add a carbonyl to the nitrogen?
Any carbonyl compound with a good or moderately good leaving group can be used to perform a nucleophilic addition–elimination reaction. These are mainly acid chlorides > anhydrides > esters.
The reaction with acid chlorides works the best since they are the most reactive derivatives of carboxylic acids. Esters, on the other hand, require heating as they are not particularly reactive. This is called the aminolysis reaction of esters, and you can read more about it in this post.
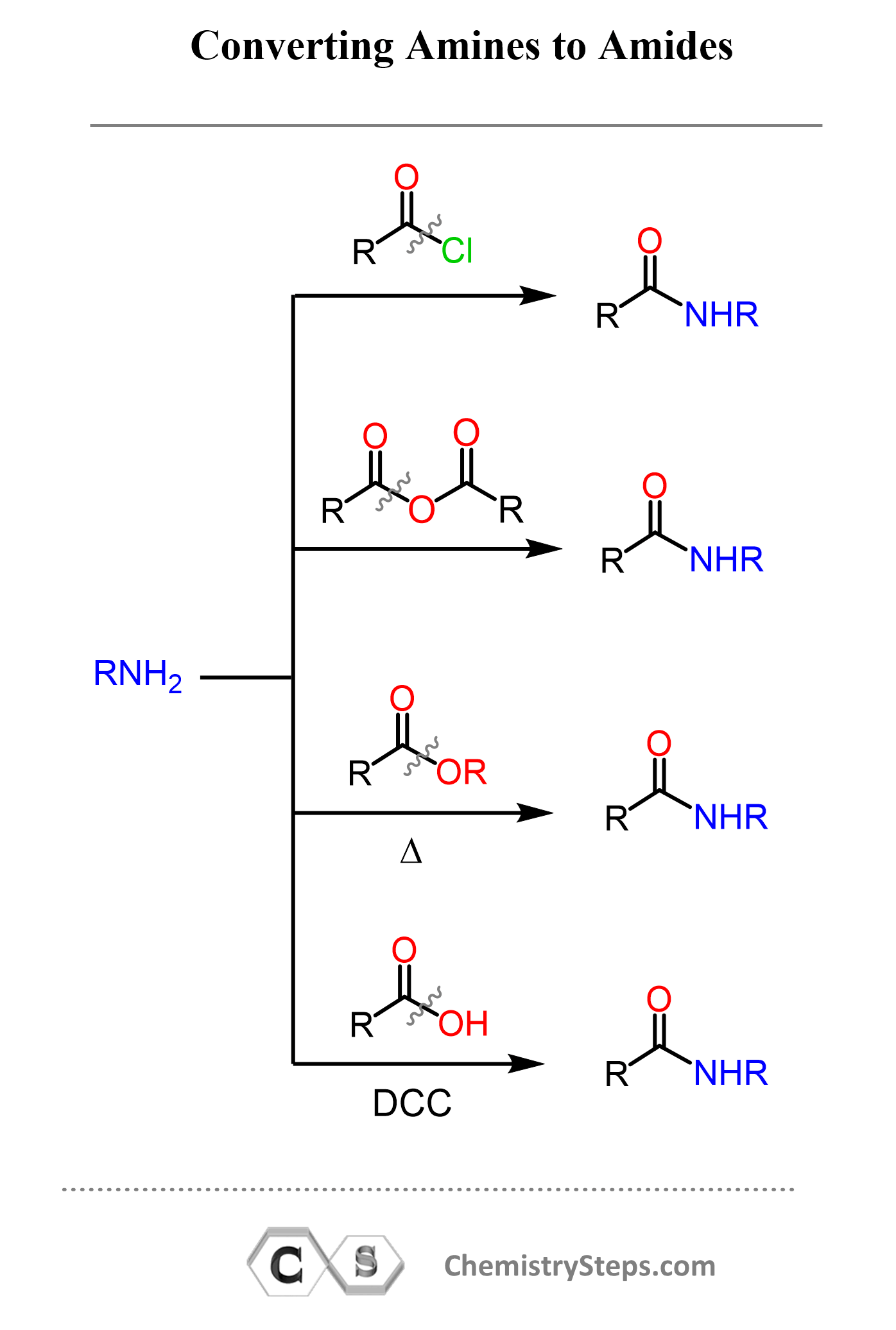
All these reactions follow the nucleophilic addition–elimination mechanism, where the amine acts as the nucleophile, the carbonyl derivative serves as the electrophile, and the group attached to the carbonyl carbon functions as the leaving group.

How easily or how difficult the substitution occurs depends on the nature of the leaving group and, of course, the amine. Primary amines are the most nucleophilic, although secondary ones work nearly as well. On the contrary, tertiary amines cannot form amides because the nitrogen does not have a hydrogen atom to be removed during the substitution step, which prevents the formation of the N–C=O bond.
The Mechanism of Amide Formation
As an example, let’s look at the mechanism of the reaction between an acid chloride and an amine. The lone pair on the nitrogen of the amine attacks the electrophilic carbonyl carbon of the acid chloride, forming a tetrahedral intermediate where the carbonyl oxygen becomes negatively charged. The nitrogen, now positively charged, is then deprotonated by a base, which is often another molecule of the amine. Next, the negatively charged oxygen reforms the C=O bond, expelling the chloride ion as the leaving group. The result is an amide, and the released HCl is neutralized by the base to form ammonium chloride.

Check this post for more details and examples of reactions between acid chlorides and nucleophiles, as well as the linked articles for each of the methods mentioned for converting amines to amides.
Amines with Carboxylic Acids
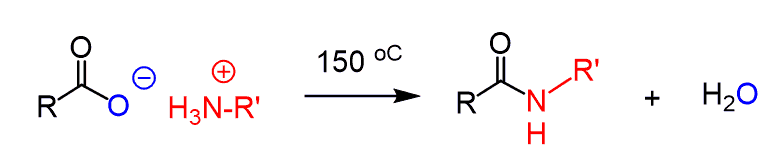
Amines can also react with carboxylic acids to form amides. We know that the –OH group is not a good leaving group, and therefore, the direct conversion requires high temperatures (usually >150 °C), where not all molecules can survive.
To perform this conversion under milder conditions, a coupling agent such as DCC (N,N′-dicyclohexylcarbodiimide) or EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) can be used. Both are carbodiimides (RN=C=NR), which prevent the acid-base reaction and make the carboxylic acid susceptible to nucleophilic attack.
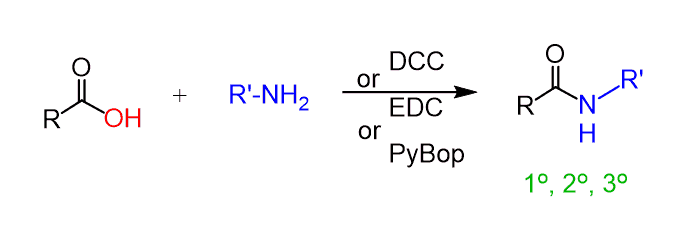
We have a separate post dedicated to the reaction between amines and carboxylic acids using different coupling reagents, so feel free to check that out as well.
Check Also
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
- Naming Amines: Systematic and Common Nomenclature
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
- The Cope elimination
- Basicity of Amines
- Boc Protecting Group for Amines