Ketones can be converted to esters through the Baeyer–Villiger oxidation, where a ketone reacts with a peracid (like mCPBA) to insert an oxygen atom next to the carbonyl, forming an ester.
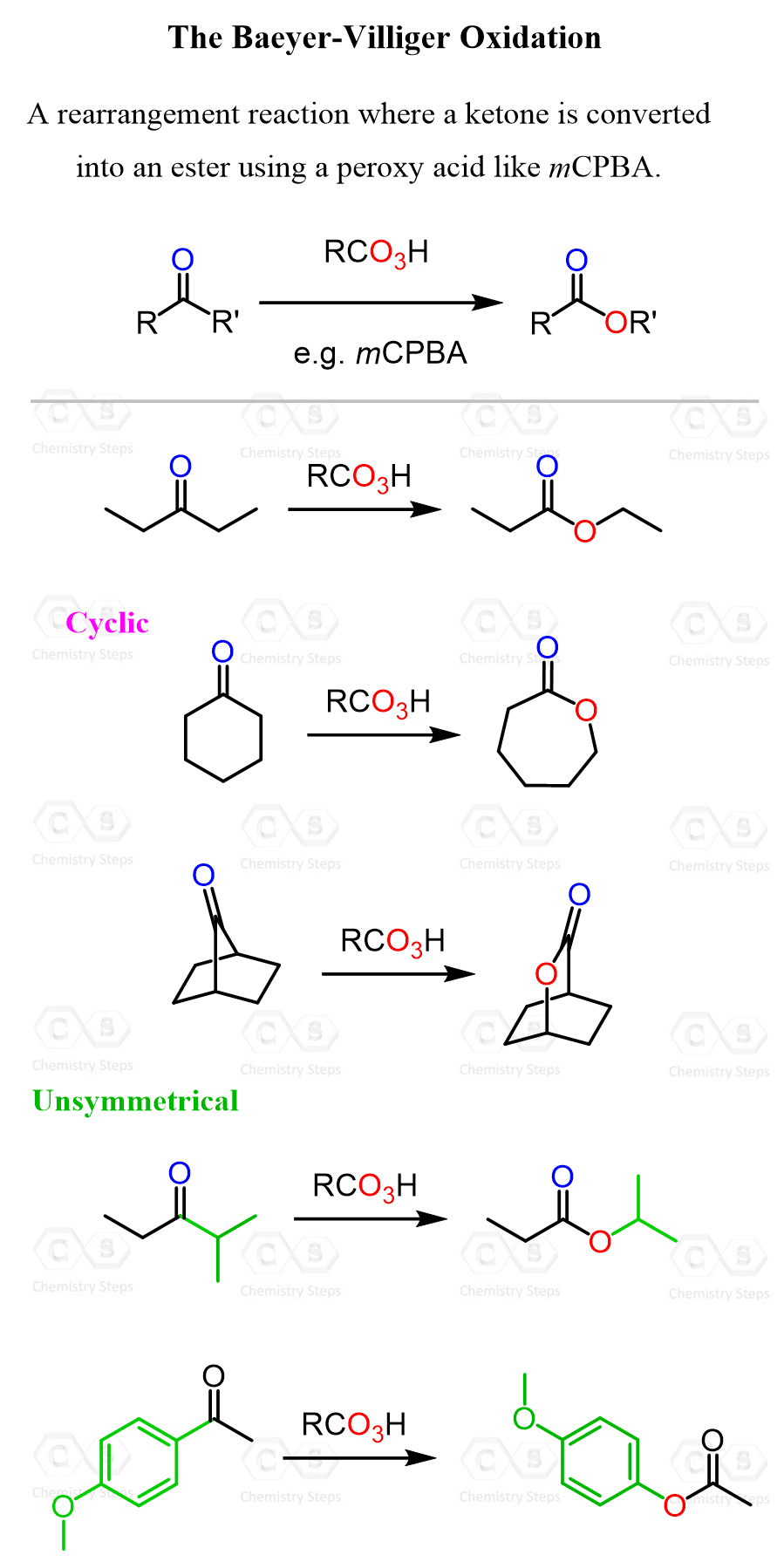
Notice that the more substituted alkyl group of the ketone ends up as the alkoxy part of the ester, due to its greater migratory aptitude. In general, the migratory aptitude in Baeyer-Villiger oxidation follows this order: H > tertiary > secondary ≈ aryl > primary > methyl.
You may be wondering what migratory aptitude has to do with this conversion of ketones to esters. Baeyer–Villiger is a rearrangement reaction where the key step involves the migration of one of the groups attached to the carbonyl carbon to an adjacent oxygen, with the group’s migratory aptitude determining the product structure:

Check the linked article for a more detailed discussion on the Baeyere-Villiger reaction.
Ketones to Esters via Carboxylic Acids
Esters are derivatives of carboxylic acids; therefore, another strategy to convert ketones to esters is by first converting them to carboxylic acids. For methyl ketones, we can use the haloform reaction. When methyl ketones are halogenated, an intermediate trihalogenated ketone is formed. The addition of hydroxide ion and subsequent expulsion of the well-stabilized -CX3 ion gives the needed carboxylic acid. The reaction is most known as iodoform (CHI3) because it is a bright yellow solid serving as an indicator for methyl ketones being oxidized to carboxylic acids in the presence of iodine:

Once we have the carboxylic acid, we can subject it to Fischer esterification. Another strategy to convert the carboxylic acid to an ester is the use of the nucleophilicity of the carboxylate ion. Carboxylic acids are very poor nucleophiles; however, if we deprotonate them, the resulting carboxylate ions are quite nucleophilic, and we can use them for SN2 substitution with alkyl halides:
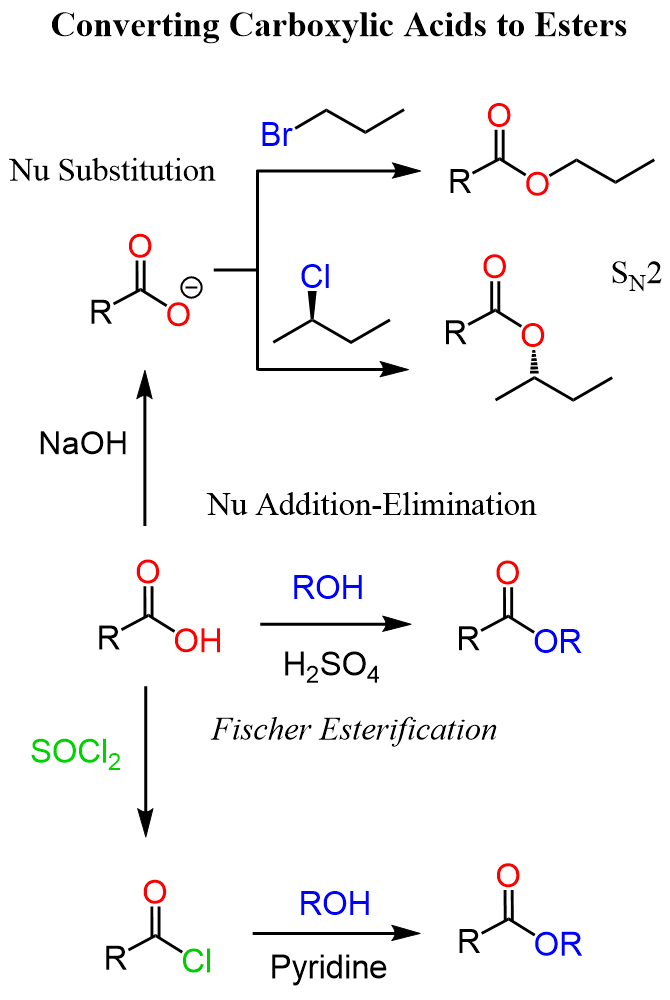
As always, please let me know in the comments if you have other methods in mind to convert ketones to esters.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Nomenclature of Aldehydes and Ketones
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- The Addition-Elimination Mechanism
- Reduction of Carbonyl Compounds by Hydride Ion
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- Reductive Amination
- Reactions of Aldehydes and Ketones with Amines-Practice Problems
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones

