There are a few ways of converting nitriles to amides. One of them is the sure way as it imposes no risk of your instructor counting it as invalid and taking some or all your points off. This is the hydrolysis of the nitrile to carboxylic acid under acidic or basic conditions at elevated temperatures followed by a condensation of the acid with an amine:

The coupling of carboxylic acid with amines is carried out using a coupling agent such as DCC or EDC. These reactions are at times challenging too, so the alternative of converting the acid to acid chloride first is also shown in the synthetic scheme. Recall that acid chlorides are the most reactive derivatives of carboxylic acids, and they readily react with all types of nucleophiles including amines.
Hydrolyzing Nitriles to Amides
The hydrolysis of nitriles to carboxylic acids goes via the formation of an amide as an intermediate.
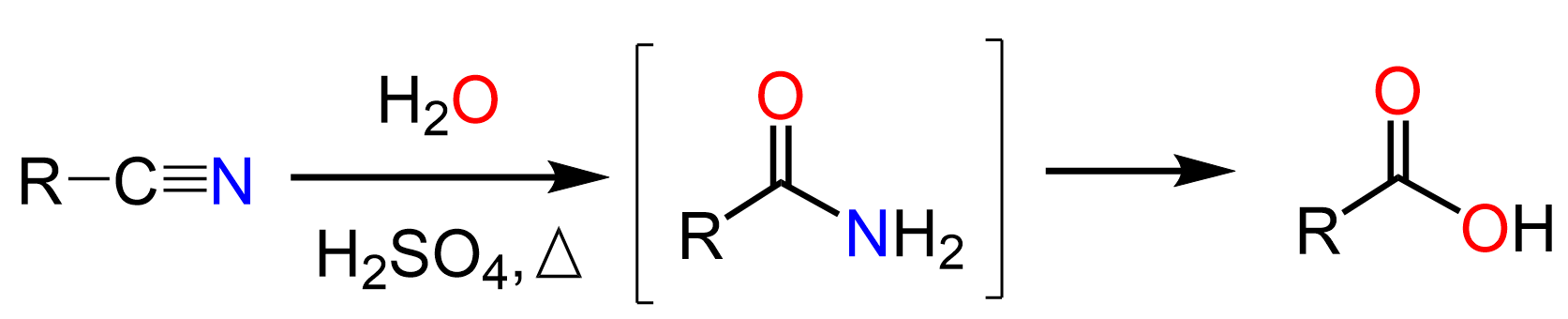
So, the question is can we stop the hydrolysis at the amide intermediate? Well, nitriles are said to be more difficult to hydrolyze than amides, and the harsh heated acidic or basic conditions hydrolyze the amide to the corresponding carboxylic acid.
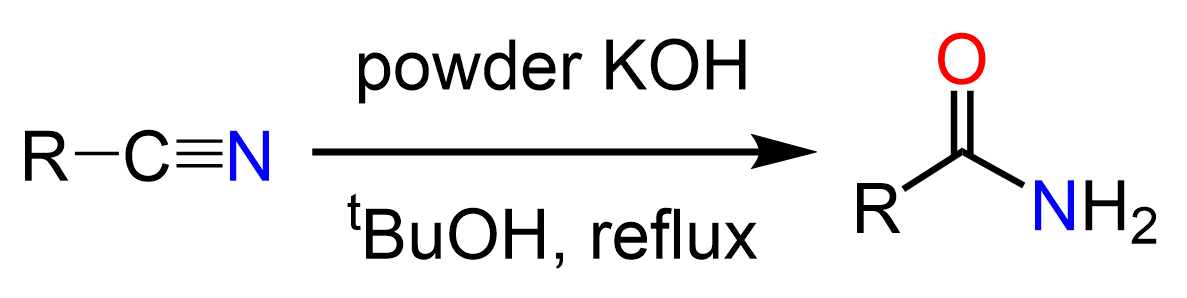
Some sources state that nitriles can be hydrolyzed to amides using milder conditions such as HCl at 40o and whether this is acceptable or not is ultimately up to your instructor. The fact is hydrolyzing nitriles to amides is not so straightforward and there is/has been research going on to find suitable conditions to achieve this transformation. For example, in J. Org. Chem., Vol. 41, No. 23, 1976, it was reported that using tert-butanol as a solvent allows to stop the hydrolysis at the amide formation.
In J. Org. Chem. 2005, 70, 1926-1929, TFA or AcOH−H2SO4 acid mixture was used for indirect hydration of the nitrile to amide. So, instead of the water acting as a nucleophile and attacking the activated nitrile, TFA does so forming another imidic intermediate which is then hydrolyzed to the corresponding amide:

The Ritter Reaction for Converting Nitriles to Amides
This is a well-known name reaction which is not included in most undergraduate organic chemistry textbooks. However, the reaction is a nice collection of protonation, activation, nucleophilic attack, and hydrolysis, and it is probably a great summary of what you have covered in your class.
So, the idea here is that the nitrile acts as a nucleophile via the lone pair of the nitrogen. Notice, it is not the –CN ion as we have seen many times in nucleophilic substitution reactions. The electrophile is a carbocation that can be formed from an alkene, an alcohol, or an alkyl halide:
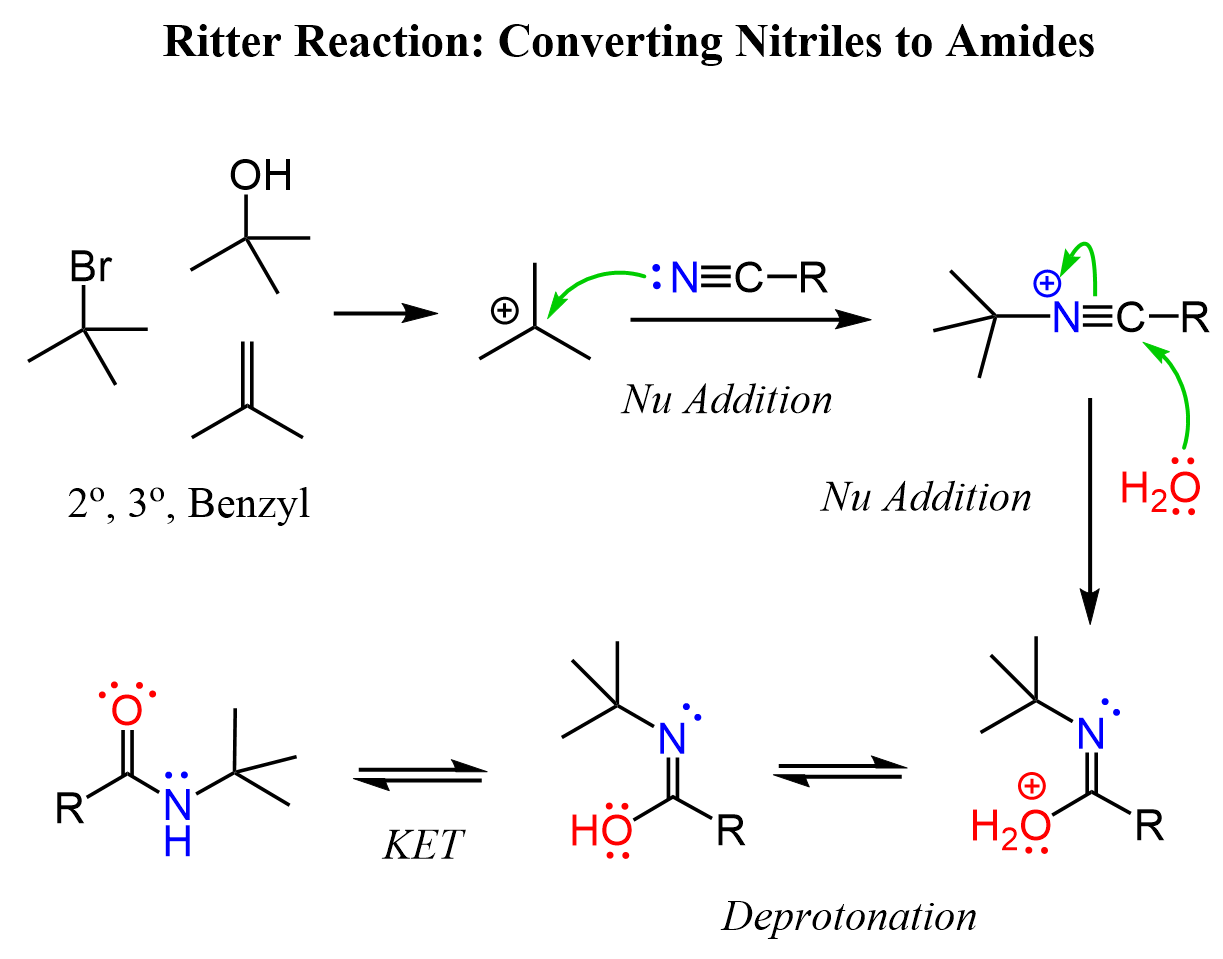
After the nucleophilic attack, the carbon-nitrogen triple bond is activated and a nucleophilic attack of water occurs as we have seen in the hydrolysis of nitriles. There is principally no difference in whether the nitrile is activated via protonation or alkylation. The nucleophilic addition of water forms an imidic acid intermediate which tautomerizes to the corresponding amide:
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz

