This is a 60-question, multiple-choice quiz covering the topics listed below.
It comes with a 1-hour video showing a detailed step-by-step solution to all the questions.
Naming Alkanes by IUPAC nomenclature Rules
First, be sure to learn the names of the first ten alkanes-methane, ethane, propane, etc…
For naming other alkanes with more complex structures, you can follow these steps:
- Identify and name the parent chain.
- Identify and name the substituents.
- Number the ring to give the substituents the smallest possible number.
- Put the substituents alphabetically followed by the parent name.
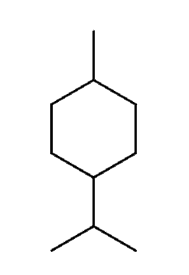
Example question:
Select the correct IUPAC name for the following compound:
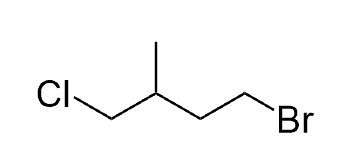
A) 1-bromo-4-chloro-3-methylbutane
B) 4-bromo-1-chloropentane
C) 4-bromo-1-chloro-2-methylbutane
D) 2-methyl-4-bromo-1-chlorobutane
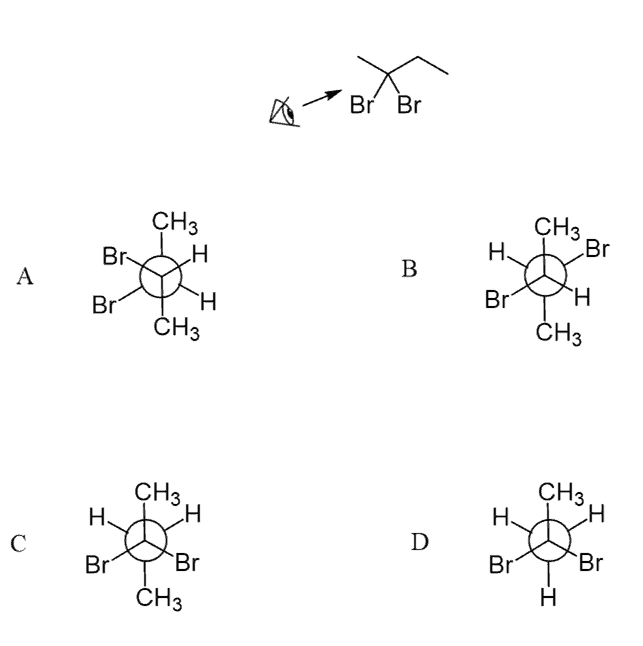
Newman Projections Gauche Conformation, Steric, Torsional Strain Energy
The questions in this quiz vary from drawing Newman projections from the corresponding bond-line structures, and vice versa, as well as determining the stability of different conformations and their energy calculations:
Select the correct Newman projection for the following compound:
Chair Conformation of Cyclohexane
Drawing a proper chair requires some practice. You can use a sheet of paper with a good chair conformation and sketch on it till you learn all the axial and equatorial positions. The questions in this quiz range from choosing the correct chair structure to recognizing ring flip and determining the more stable chair conformation.
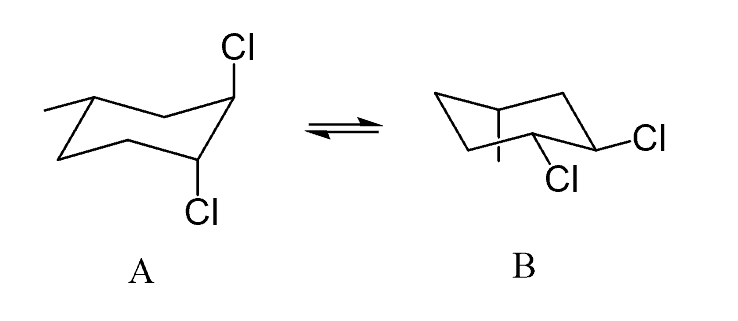
Identify the correct representation of the ring flip for the following compound:

Ring Flip: Drawing Both Chair Conformations
1,3-Diaxial Interactions and A value for Cyclohexanes
Use the table to calculate the relative energy cost associated with each group in the axial position to determine the more stable chair conformation of each of the following compounds:

Ring-Flip: Comparing the Stability of Chair Conformations
Cis and Trans Isomerism in Cyclohexanes
Draw the cis and trans isomers of the following compound then convert them into the chair representations to determine the more stable (cis or trans) isomer: