This is a multiple-choice quiz to practice the Electrophilic Addition Reactions to Alkenes
It comes with a detailed, 3-hour video solution addressing all the correct and incorrect answers!
Markovnikov’s Rule
The Stereochemistry of Alkene Addition Reactions
Free-Radical Addition of HBr: Anti-Markovnikov Addition
Acid-Catalyzed Hydration of Alkenes
Oxymercuration-Demercuration
Hydroboration-Oxidation: The Mechanism
Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
Halogenation of Alkenes and Halohydrin Formation
Ozonolysis of Alkenes with Practice Problems
Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
Anti-Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
Oxidative Cleavage of Alkenes with KMno4 and O3
Cis product in an anti Addition Reaction of Alkenes
Alkene Reactions Practice Problems
Changing the Position of a Double Bond
Changing the Position of a Leaving Group
Alkenes Multi-Step Synthesis
Addition of Alcohols to Alkenes
Below are some example questions:
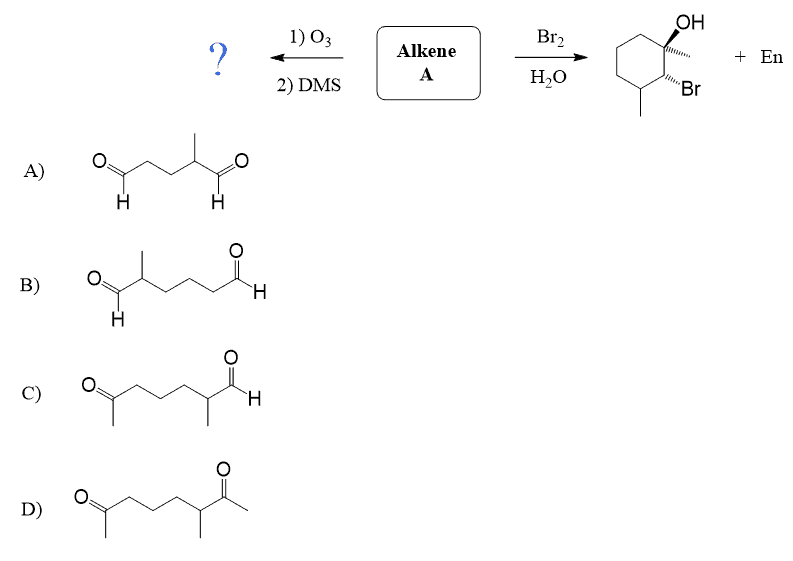
Identify the structure of the unknown based on the two reactions provided below:
Determine the major product(s) of the following reaction:



Where are the hints or direct link to get help for these questions. Do you have explanation of the correct answers? If so, where?
Currently working on them. They should be there by the end of the week. All the topics in the quiz are covered in the Alkenes chapter which you can find on the index page:
https://www.chemistrysteps.com/organic-chemistry-topic-index/
Is there a particular one you had a question about?
The link helps thank you.
Great!
the answer for #18 is wrong, it should be D not C
It is C. Draw the alkene and cross each double bond replacing it with a carbonyl. You should get three products – A, B, and D. I will try to find a better way of showing this but for now, consider the left carbonyl in product C – it cannot be formed.
And here is the better way of showing it. I hope it helps:
would the answer for #39 be C not B, since H2O attacks from the back (opp side of the triangle O structure thing) to create anti OHs?
or were they just rotated to make both OH bonds look the same since it’s a meso compound?
Your considerations about the stereochemistry of the anti-hydroxylation are absolutely correct. However, you should also pay attention to the stereochemistry of the alkene. Here, we start with a cis alkene and if you draw the OH groups as trans you should also keep the “bent” geometry of the carbon chain. If you convert this into the more traditional zig-zag conformation, the OH groups now end up on the same side and it looks like it is a product of a sun-dihydroxylation, but it is not.
This is a bit of a trick question that may become handy on your test.
Find this article on the index page which explains this in more detail:
“Cis product in an anti Addition Reaction of Alkenes“
They were indeed rotated, but this is not a meso compound since there is no plane of symmetry. You can visualize this by rotating it back again – the OH groups are wedge and dash, meaning they are not reflected through a mirror.
You can also check this by the R and S configuration; in a meso compound every S is converted to an R and vise versa.
The meso compound would have been produced if a syn dihydroxylation was performed.
I covered this in the post about meso compounds:
“Symmetry and Chirality. Meso Compounds” on the index
Specifically, go to the “Wedge and dash in Meso compounds” paragraph.
if the alkene was originally trans not cis, then the backside attack of H2O after the epoxide forms would create a molecule where if u rotate it, it would look like B. but since the alkene was cis to start with, i think the backside attack of H2O after the epoxide forms would yield C
For question 34, I thought bromination is an anti-addition, so on the Fischer projection the Br atoms should point away from each other…?
Yes, that is correct. However, you need to keep in mind that in Fischer projections, the carbon chain is not shown in a zig-zag form, but rather it is bent such that the top and bottom carbons are pointing away from the viewer. When you convert the product of anti addition to a Fischer projection, the Br atoms then appear to be on the same side.

Check articles mentioned in the hint and also this one specifically about the cis and trans products in the addition reactions of alkenes:
https://www.chemistrysteps.com/cis-product-anti-addition-reaction-alkene/