In the previous post, we talked about the Hofmann Rearrangement, which is the conversion of primary amides to primary amines:
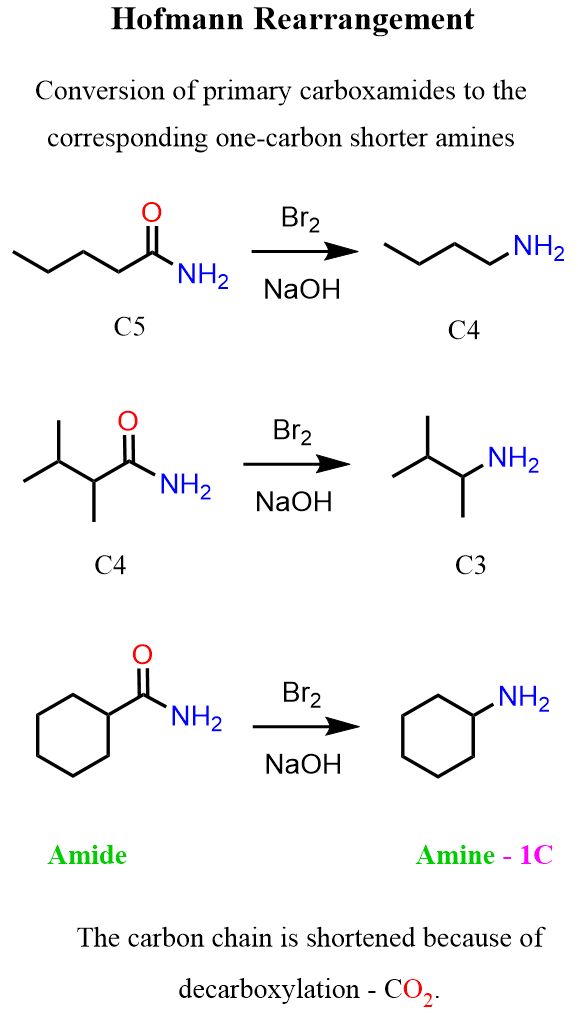
Notice that the carbon chain of the product is shorter by one atom because of the decarboxylation that the carbamic acid intermediate undergoes. A key step and intermediate in this reaction is the 1,2-alkyl/aryl shift, which converts the N-bromoamide into an isocyanate (R–N=C=O). Feel free to check the post on the Hofmann rearrangement to refresh some of these patterns, because the Curtius rearrangement is very similar to it.
The Curtius rearrangement is very similar to the Hoffman rearrangement. The difference is that here, the starting material is an acyl azide rather than an amide.
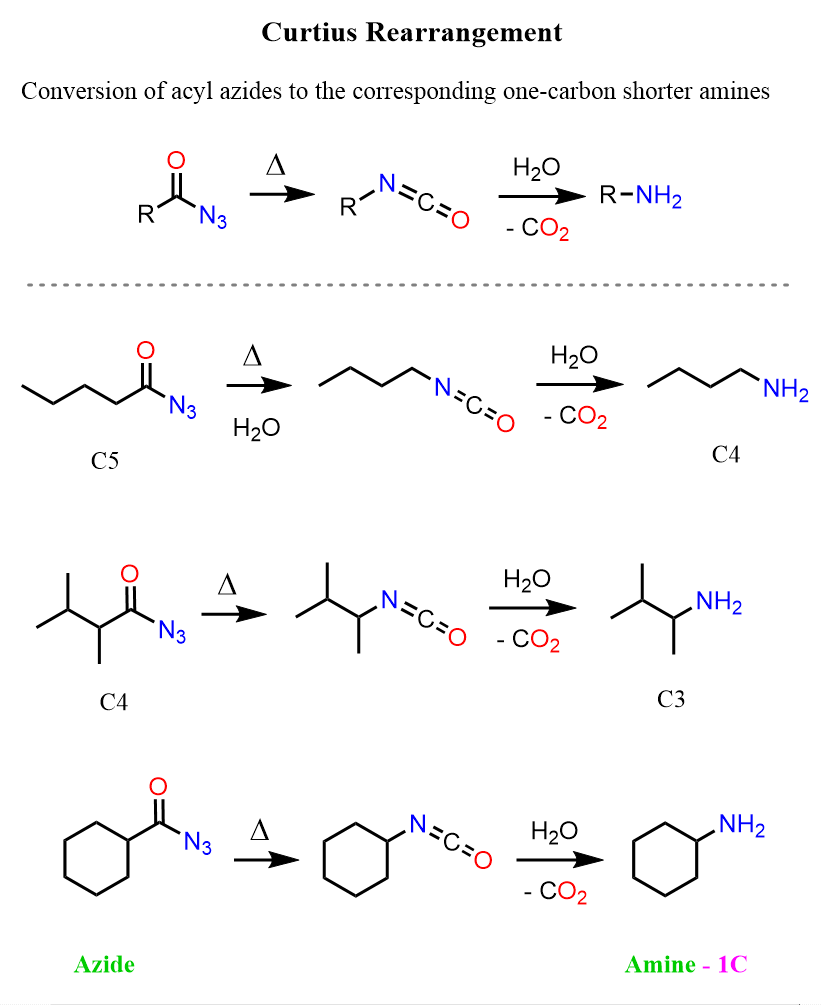
Like in the Hofmann reaction, the key intermediate here is the isocyanate that is formed via a 1,2-shift, which, this time, is facilitated by the presence of an exceptional leaving group nitrogen. It is arguably the best leaving group as it is hard to imagine a more stable one, and in addition, being a gas, the formation of nitrogen is entropically very favorable as well. We have seen it making the formation of phenyl cations/radicals possible in the reaction of arene diazonium salts. Recall that the phenyl cation is not resonance-stabilized and very unstable, contrary to what it may look like.
Ok, back to the Curtius rearrangement: Aside from the formation of the isocyanate intermediate, the other steps should be fairly intuitive if you spend some time studyingand practicing the reactions of carboxylic acid derivatives. So, let’s now summarize the mechanism of Curtius rearrangement:
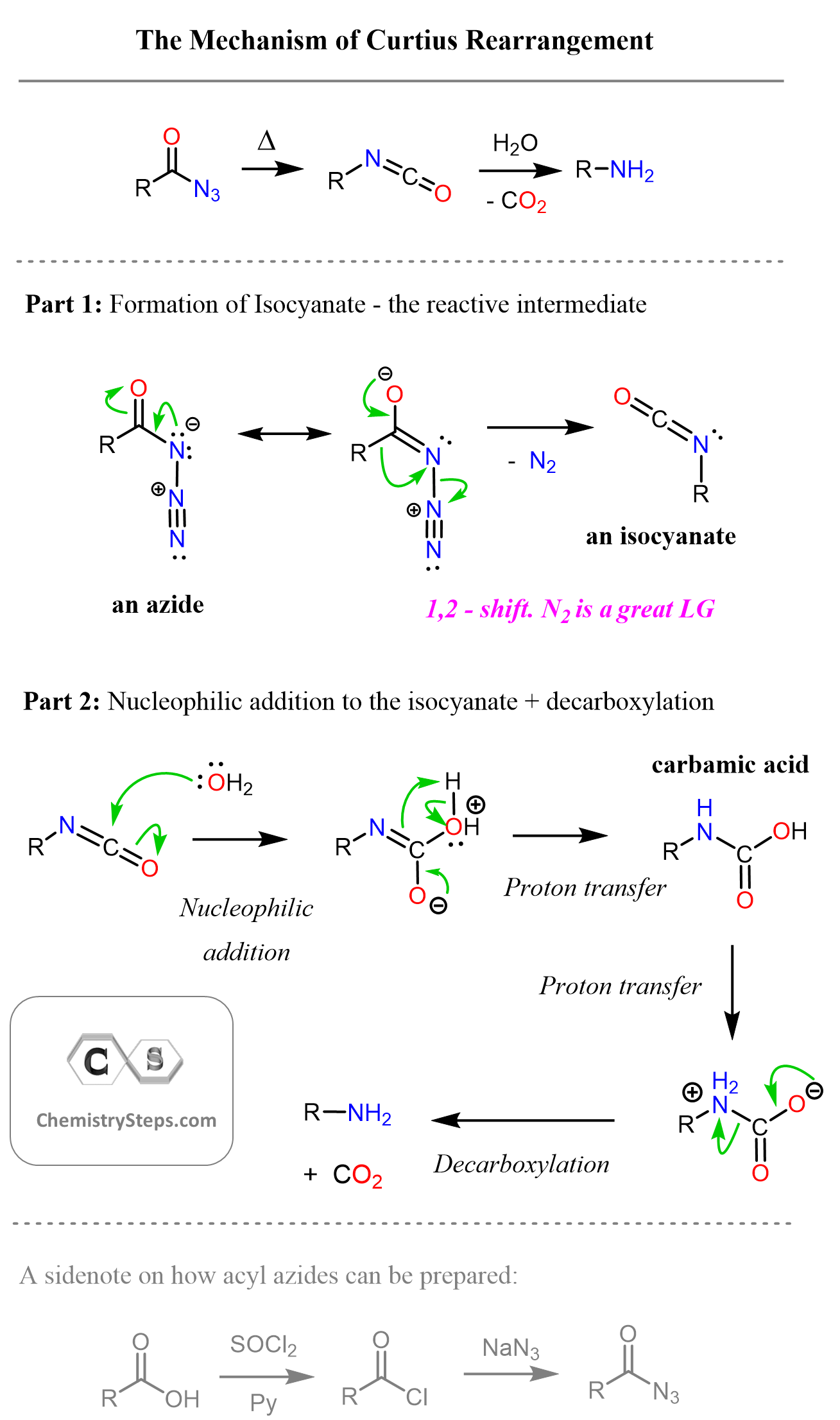
Depending on the reaction conditions, the isocyanate can be first protonated to activate the carbonyl group, or it can be attacked by a hydroxide ion if run in basic conditions.
Acyl azides can be prepared from carboxylic acids by first converting them to acyl chlorides using thionyl chloride (SOCl2) and an organic base such a pyridine (Py) or triethylamine (TEA), etc.
Comparing the Hofmann and Curtius Rearrangements
As mentioned above, both these reactions proceed via the same isocyanate intermediate, and the difference is the starting material. This little chart should help you visualize and compare the Hofmann and Curtius rearrangements:
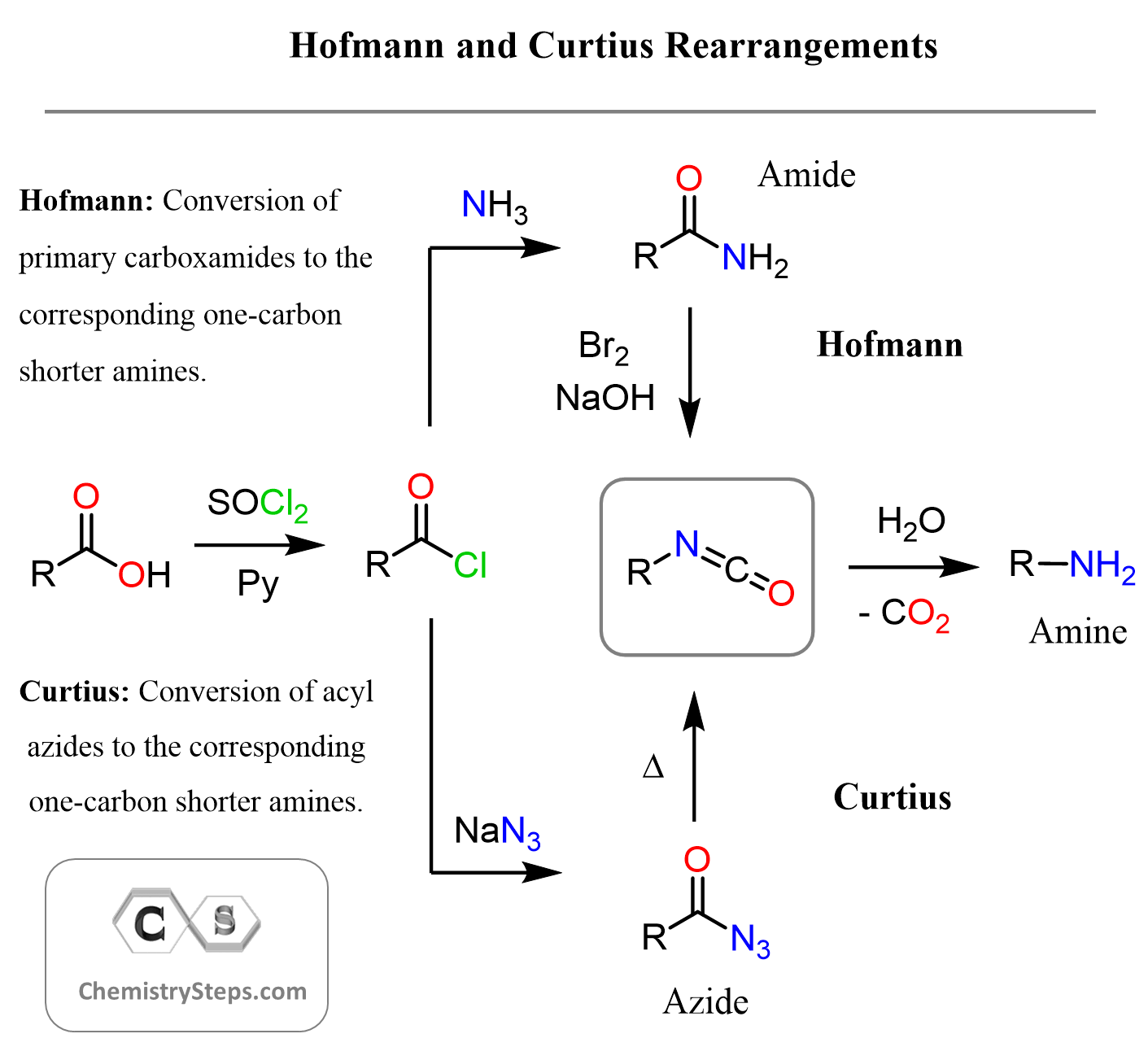
This is what I wanted to have as a review of the Curtius rearrangement and its comparison to the analogous Hofmann reaction. Let me know in the comments if you have any questions, and check out the collection of practice problems on the reactions of carboxylic acid derivatives.
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
