Decarboxylation is the loss of carbon dioxide upon heating, typically characteristic of carboxylic acids with a β-carbonyl group. These are most often beta-keto acids, malonic acid, or its derivatives:

Carboxylic acids by themselves are very stable and do not undergo decarboxylation even at their boiling points. So, what we need for decarboxylation is the carboxylic group and a carbonyl separated by one carbon atom. The question is then, what is the role of the carbonyl group that facilitates the decarboxylation?
To answer this question, we need to look at the mechanism of decarboxylation. It is a concerted mechanism that passes through a six-membered transition state in which the carbonyl serves as an acceptor for the electrons of the breaking C-C bond:

Decarboxylation in Acetoacetic Ester Synthesis
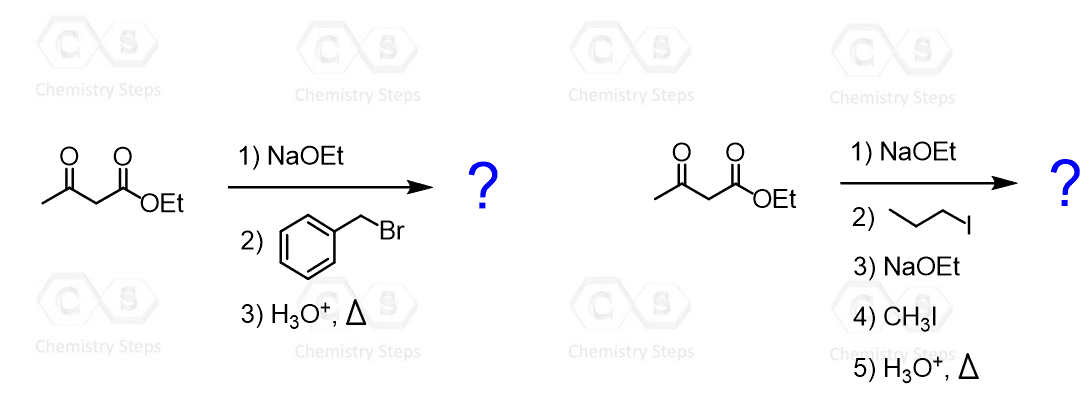
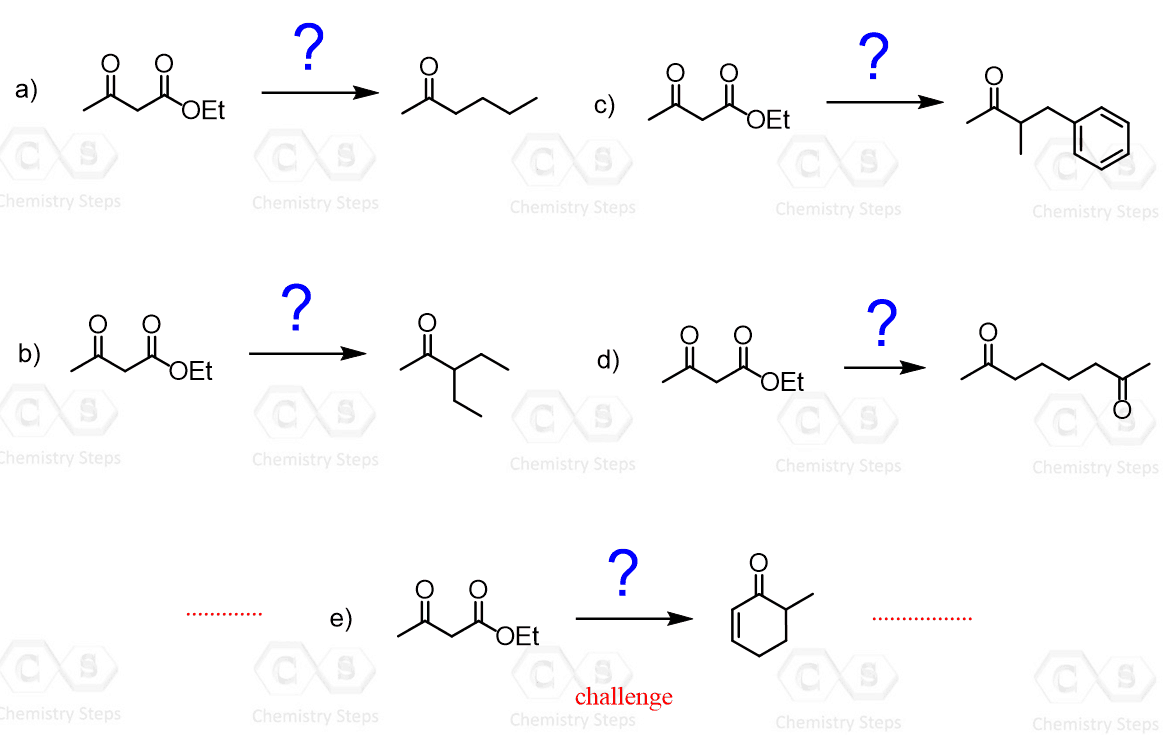
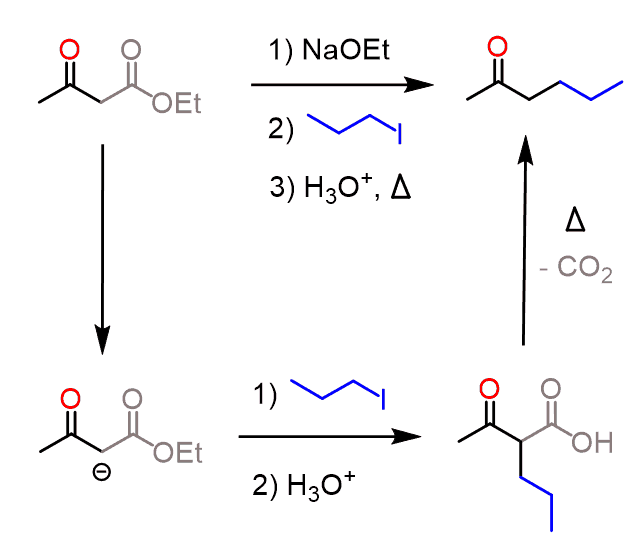
Some common uses of decarboxylation are those of malonic ester and acetoacetic ester. The acetoacetic ester synthesis is a useful synthetic tool for preparing ketones having one or two alkyl groups on the ɑ position:
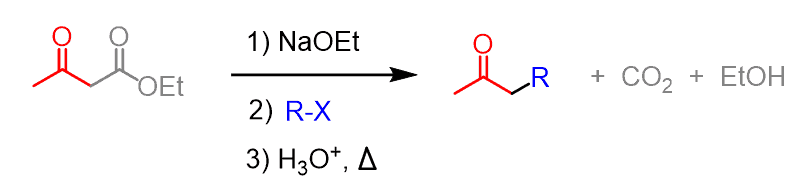
In the first step, acetoacetic ester is deprotonated and can then serve as a nucleophile and attack the alkyl halide. After this, the ester is then hydrolyzed to a carboxylic acid, which then loses carbon dioxide through a nicely arranged six-membered transition state:

The resulting enolate tautomerizes to a ketone with a newly incorporated alkyl group on the ɑ position. For example, here is how 2-hexanone can be synthesized from acetoacetic ester:
Decarboxylation in Malonic Ester Synthesis
A similar transformation can be achieved by using the malonic ester synthesis. The only difference is that the final product is a carboxylic acid instead of the ketone obtained in the acetoacetic ester synthesis:

For example, alkylating malonic acid with 1-iodopropane results in pentanoic acid, while acetoacetic ester gives 2-hexanone:

Once again, the decarboxylation occurs through a nicely arranged six-membered transition state after the ester is hydrolyzed to the corresponding acid:

Decarboxylation in Claisen Reactions
Esters are known to undergo an aldol-like reaction called Claisen Condensation since they too have an acidic ɑ position to form an enolate and, of course, a carbonyl to serve as an electrophile. The product in the Claisen condensation is a β-keto ester, and therefore, we can hydrolyze it to an acid and decarboxylate further to a ketone.

Here is a practice example to convert the nitrile to a ketone, which involves Claisen condensation and decarboxylation:
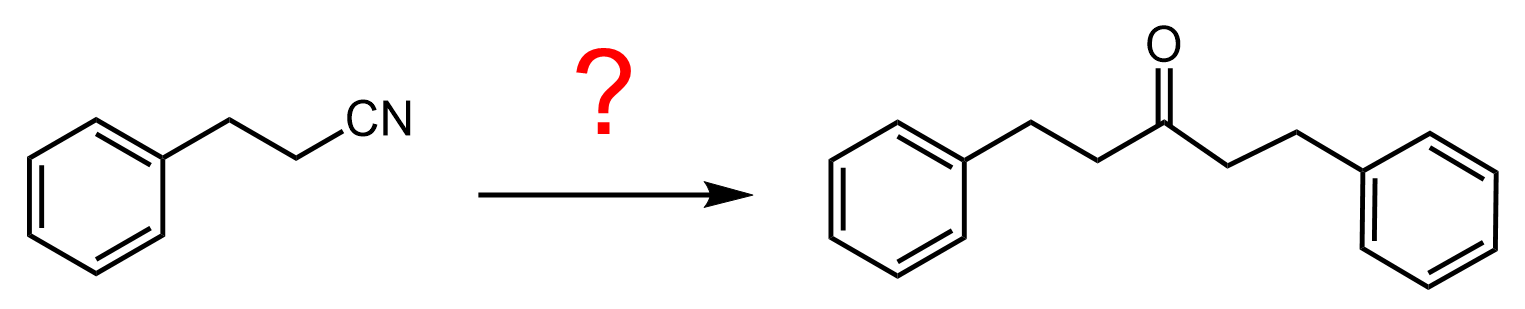
The answer is given in the practice problems section.
Decarboxylation in Cleaving the Boc Protecting Group
Boc (tert–butyloxycarbonyl) is the most common protecting group for amines, and its functional features, aside from being stable under basic conditions, are explained by the stability of the t-butyl carbocation and decarboxylation of the intermediate carbamic acid. Below is the summary of the mechanisms of adding and removing the Boc group:

You can find more about the protection of mines using Bloc here, and meanwhile, here are some practice examples involving decarboxylation.