We have seen earlier how alkyl halides undergo E1 and E2 elimination reactions to form alkenes:

In those reactions, the leaving group was the halide, which was kicked out by removing the β-hydrogen and making a new π bond.
Somewhat like this, alcohols also undergo a β elimination reaction called dehydration (loss of a water molecule) – in which the elements of OH and H are removed to form an alkene:

Dehydration of alcohols requires a strong acid and is carried out at high temperatures (100-200 oC). The most common strong acid used for dehydration is concentrated sulfuric acid, even though phosphoric acid and p-toluenesulfonic acid (abbreviated as TsOH) are often used as well.
The reaction can follow both E1 and E2 mechanisms depending on whether it is a primary, secondary, or tertiary alcohol.
Dehydration of Tertiary and Secondary Alcohols Follows the E1 Mechanism
Let’s start with tertiary alcohols, which follow the E1 mechanism:

The first step of the reaction is the protonation of the hydroxyl group, which converts the OH into a good leaving group by weakening the C-O bond:
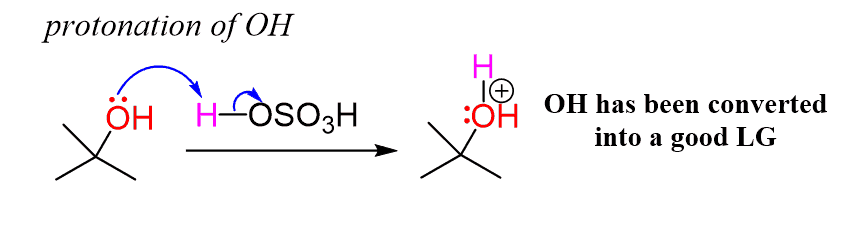
Notice that, unlike the dilute sulfuric acid, where the protons exist mainly as hydronium ions, the concentrated sulfuric acid is the proton donor here.
The protonated alcohol is the substrate that undergoes an E1 elimination, which, remember, starts with the loss of the leaving group:
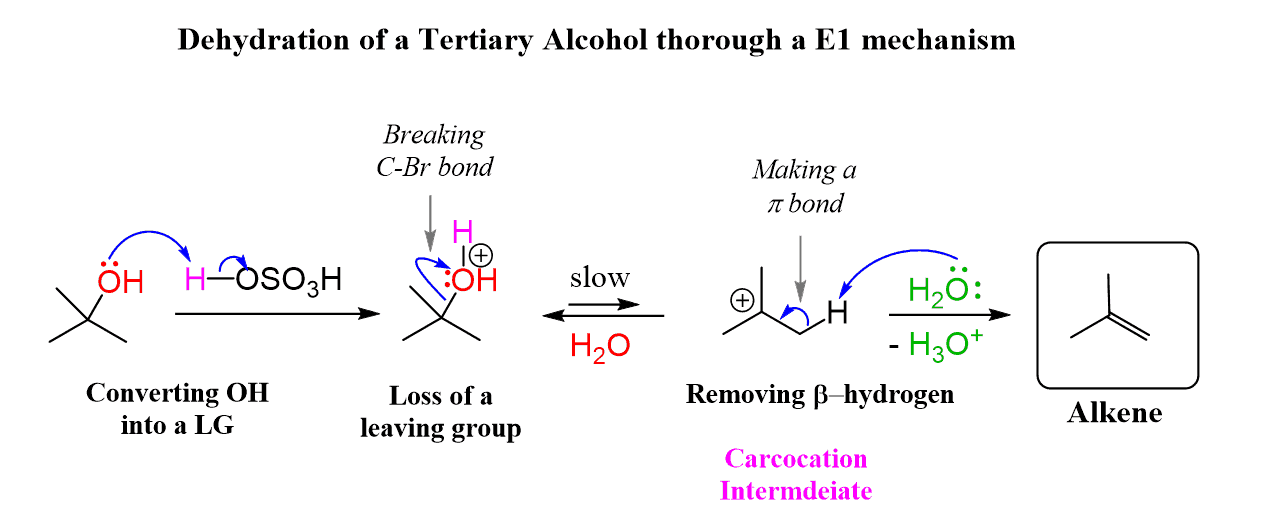
The loss of the leaving group is a heterolytic cleavage of the C-O bond, and as expected, it is the rate-determining step of the reaction. Therefore, this step determines the overall reactivity of alcohols in dehydration reactions.
And just like in SN1 and E1 reactions, tertiary substrates tend to be the most reactive because of the stability of the corresponding carbocations.
Remember, more substituted carbocations are more stable because of the hyperconjugation and electron-donating nature of alkyl groups.
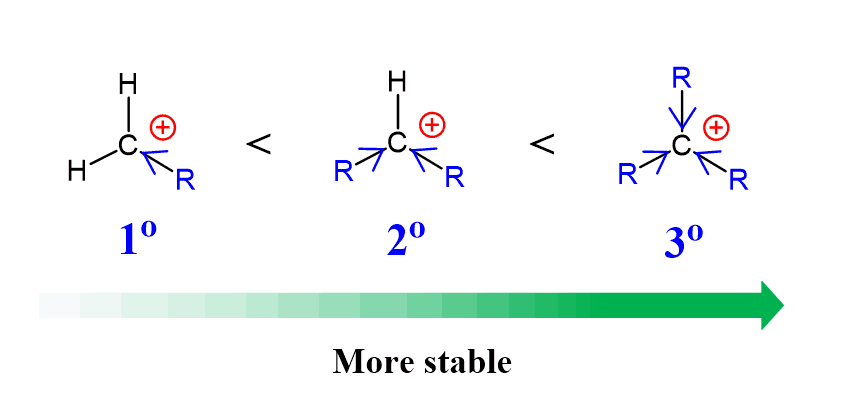
The reactivity trend in dehydration reactions can be illustrated by the transition state of this step, where the relative free energies of activation are tertiary < secondary < primary:

The carbocation formed after the loss of the leaving group is very reactive because the central carbon atom lacks an octet, and the water now acts as a base, removing the β-hydrogen to donate an electron pair. The electron pair from the proton forms the π bond of the alkene.
Because of the stability of tertiary carbocations, tertiary alcohols are the easiest to dehydrate, and even 30% aqueous sulfuric acid can be used at temperatures below 100 °C.
Dehydration of Secondary Alcohols
Secondary alcohols require more concentrated acid solutions and higher temperatures. For example, cyclohexanol is dehydrated to form cyclohexene using concentrated sulfuric acid at 160–180 °C:

The reaction still goes by the E1 mechanism, and the rate depends on the stability of the secondary carbocation.
Regioselectivity of Dehydration Reactions
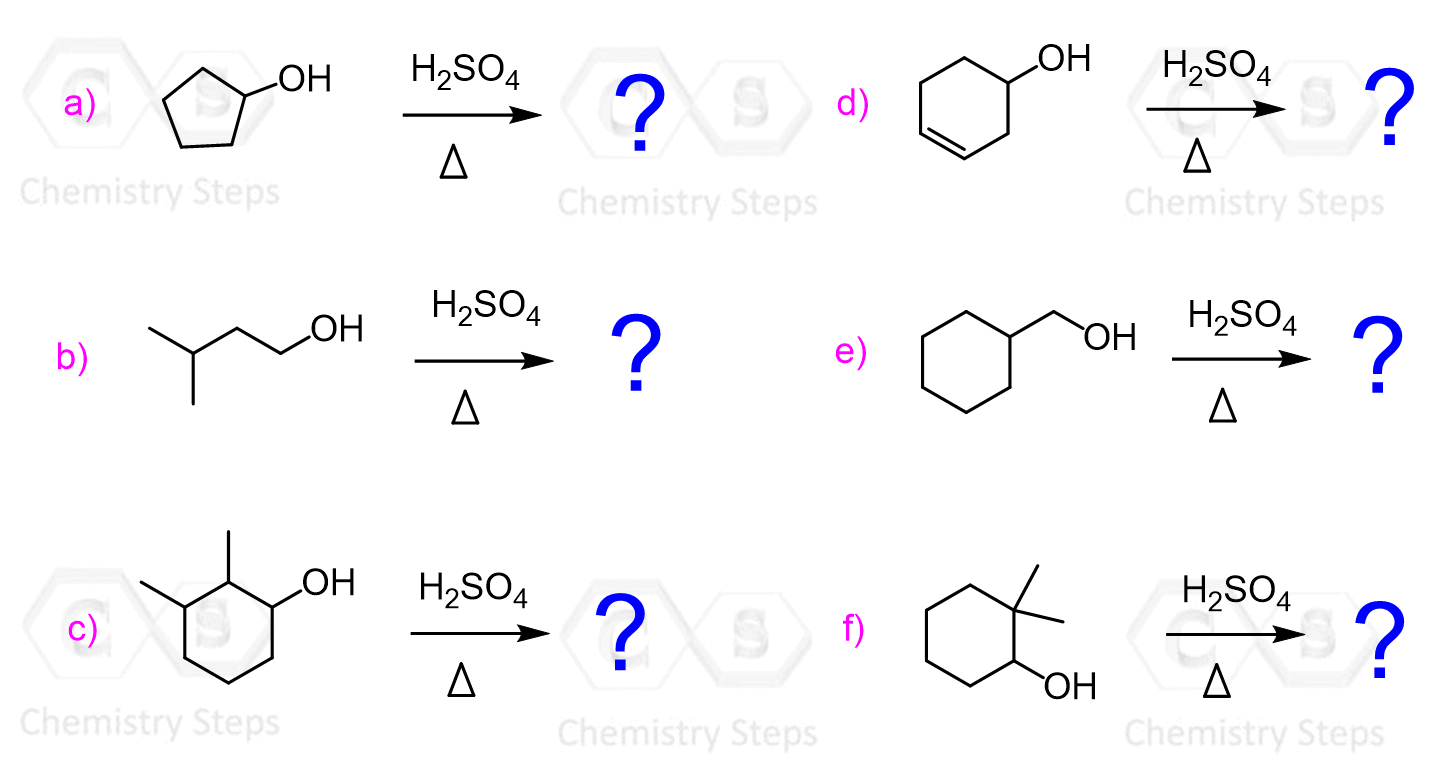
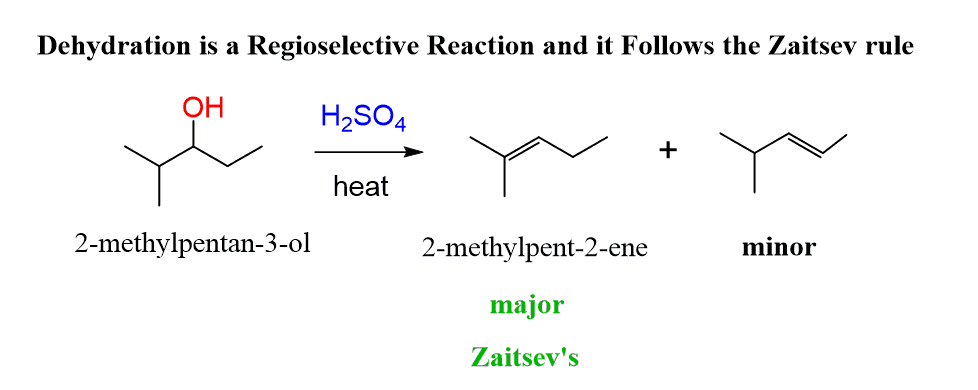
The dehydration is a regioselective reaction, and it follows Zaitsev’s rule. The more substituted alkene is the major product when a mixture of constitutional isomers is possible.
For example, dehydration of 2-methyl-3-pentanol produces the more substituted 2-methylpent-2-ene as the major product:
Rearrangements in Dehydration Reactions
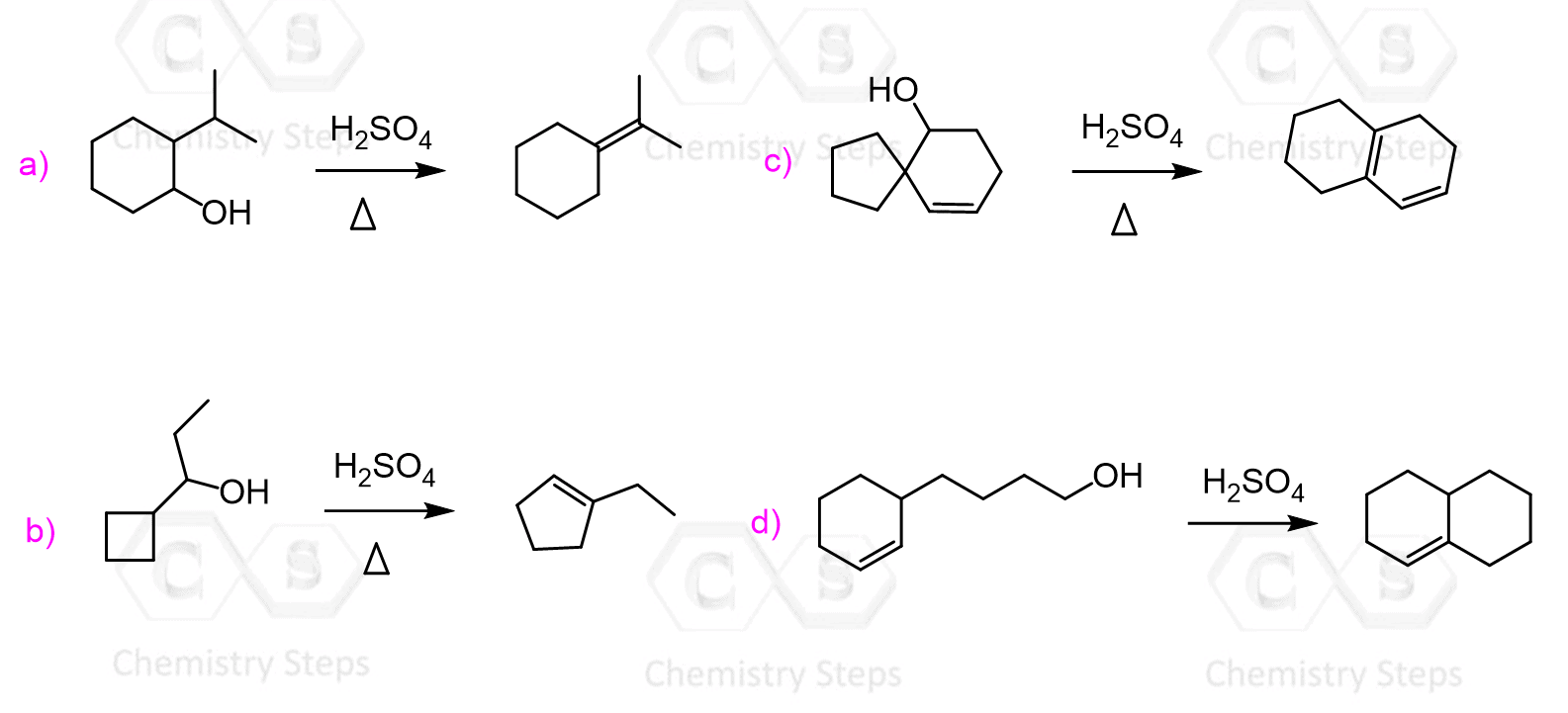
Even though dehydration of alcohols is regioselective, you should always watch for rearrangements.
We have learned that rearrangements of the carbocation in SN1 and E1 can occur, and dehydration is not different:

Whenever a more stable carbocation can be formed, you should expect a rearrangement of the carbon skeleton.
For example, the following alcohol is expected to form a trisubstituted alkene as the major product when treated with concentrated sulfuric acid:

The major product, however, is a tetrasubstituted alkene which is formed as a result of a hydride shift to transform the secondary carbocation into a more stable tertiary carbocation:

The E2 Mechanism of Dehydration of Primary Alcohols
Primary alcohols react the slowest in dehydration reactions.
The reaction proceeds through an E2 mechanism because primary carbocations are highly unstable and cannot be formed as they do for secondary and tertiary alcohols:
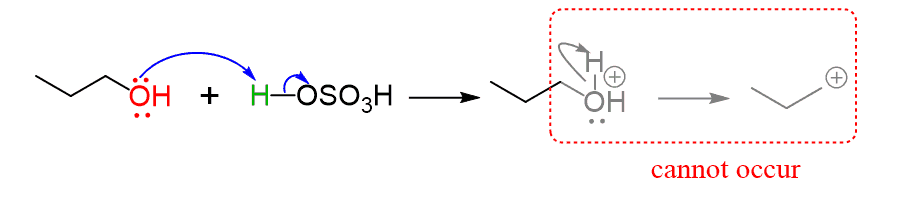
There are some similarities in these reactions, and just like in the E1 mechanism, the dehydration starts with the protonation of the primary alcohol, turning it into a good leaving group:
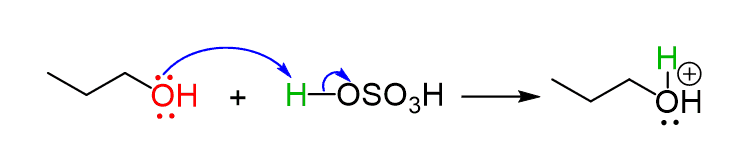
The difference with the E1 mechanism is that there is no loss of a leaving group happening by itself, as this would form a primary carbocation.
Instead, the base (water of bisulfate ion) attacks now the β hydrogen, which leaves a pair of electrons kicking out the protonated OH group and making a double bond:

Notice that these processes happen simultaneously, and that is why it is a bimolecular – E2 mechanism.
Rearrangements in E2 Dehydration of Alcohols
Rearrangements in E2?!
Yes, you read that right.
Let’s discuss the dehydration of the following primary alcohol:
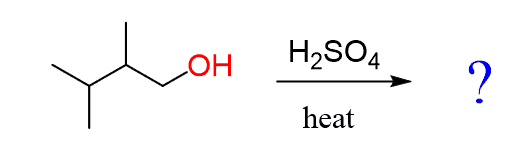
How do we explain the formation of a tetrasubstituted alkene as the major product of this reaction?
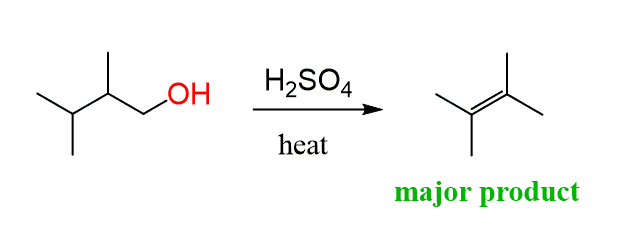
It is a primary alcohol, so no primary carbocation can be formed; therefore, a carbocation rearrangement does not explain this observation.
What happens here is, after the protonation of the OH group, a hydride shift from the β carbon to the terminal carbon of the primary alcohol,l kicking out the excellent leaving group water.

So, unlike the rearrangements of carbocations that we have seen before, where the loss of the leaving group happens before the hydride or a methyl shift, here the shift happens while the leaving group is connected. In fact, it is the shift that kicks out the leaving group.
This hydride shift produces a relatively stable secondary carbocation, which then is attacked by a base to form the more substituted alkene according to the Zaitsev’s rule:
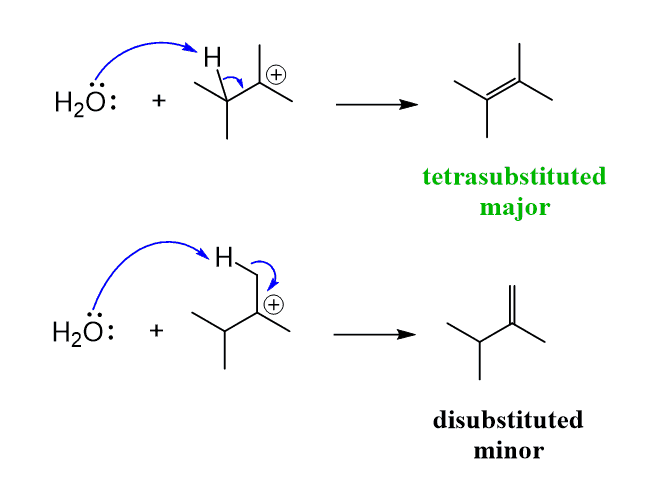
Another possibility of forming this alkene is explained by the reversible nature of the dehydration reaction.
The thing is that alkenes react with water in the presence of acid catalysts. This is known as the acid-catalyzed hydration of alkenes:

You may not have covered this in your class, but we will show the mechanism quickly to give a basis for understanding the formation of the tetrasubstituted alkene in the dehydration reaction discussed above.
The reaction starts by protonation of the double bond, forming a carbocation, which is then attacked by water:

The water serves here as a nucleophile similar to the SN1 reaction.
So, if we pay closer attention, both reactions are performed in acidic solutions, and the only difference is the concentration of the acid. Dehydration is achieved in concentrated acids, while acid-catalyzed hydration is performed in dilute acidic solutions:
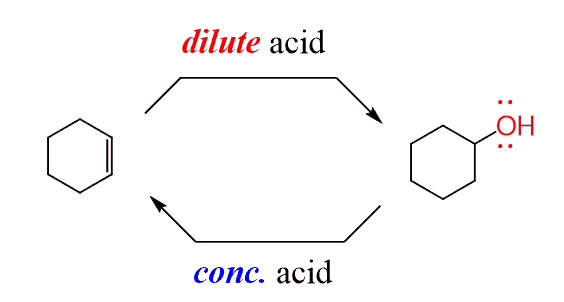
Now, going back to the dehydration. Let’s, for a moment, forget about the hydride shift that we discussed for the reaction of 1-propanol and explain the formation of the tetrasubstituted alkene using the reversible nature of the dehydration.
Again, if there was no hydride shift, this primary alcohol would have formed a disubstituted alkene according to the E2 mechanism we discussed for propanol:

This alkene can now be protonated to form a tertiary carbocation:
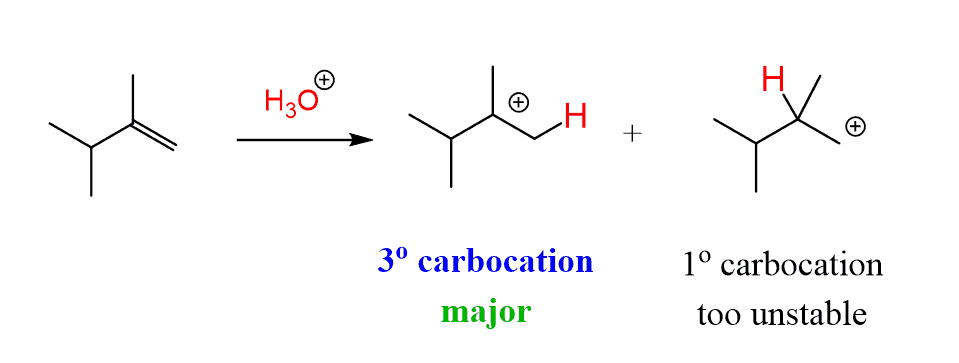
From here, there are two possible products of removing a β-hydrogen: a disubstituted alkene, which is the reverse reaction of the protonation, or a tetrasubstituted alkene, which is more stable and predominates as expected:

In a short summary, dehydration of a primary alcohol can be shown by including the reversible step of protonation of the double bond, thus forming a more stable carbocation, which leads to a more stable, internal alkene:
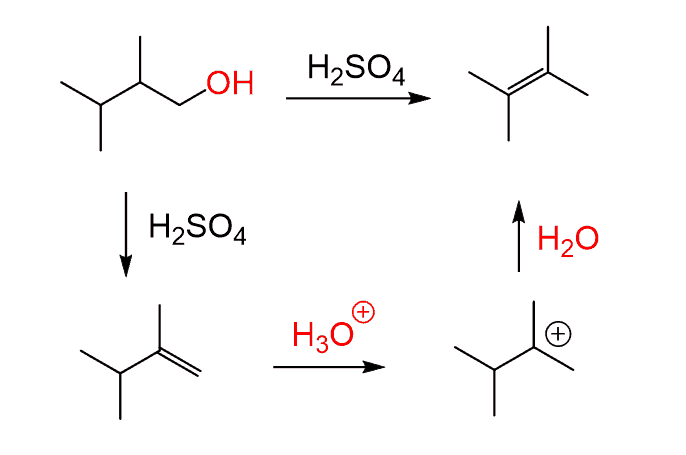
Another approach for the dehydration of alcohols is the use of POCl3 in the presence of a base. This is a great alternative since it follows an E2 mechanism for 1o, 2o, and 3o alcohols, thus avoiding any rearrangements.

All the details for this reaction are covered in the following post:
POCl3 for Dehydration of Alcohols
Was that it?
Sorry, this is not the only complication we see in the dehydration of alcohols. One more side reaction to take a look at:
SN2 During Dehydration of Alkenes
The protonated form of the hydroxyl group is an excellent leaving group, and when it is a primary alcohol, there is a possibility of an SN2 reaction to form an ether:
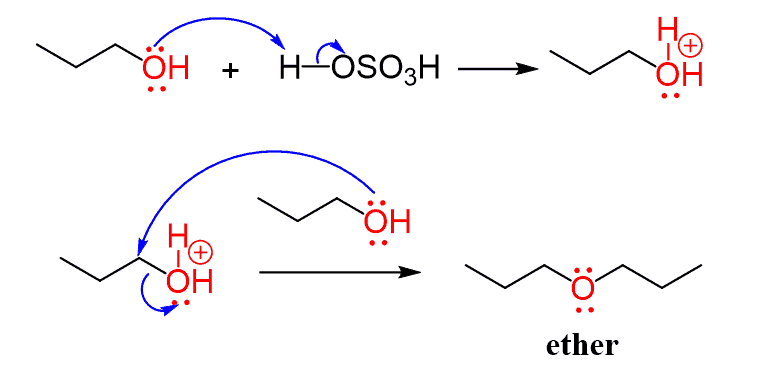
However, the good news is that, under the high-temperature conditions, elimination reactions predominate, and the major product of reacting an alcohol in a concentrated acidic solution is the alkenes rather than the substitution products.
The reason for favoring elimination over substitution at elevated temperature has to do with the entropy of these reactions. It is covered in more detail under the section “Why does Heat Favor Elimination? in the E1 mechanism.
Check out this 65-question, Multiple-Choice Quiz with a 3-hour Video Solution covering Nucleophilic Substitution and Elimination Reactions:
Nucleophilic Substitution and Elimination Practice Quiz