Diisobutylaluminium hydride also known as (DIBAL, DIBALH, DIBAL-H or DIBAH) is a bulky reducing agent that is mainly used for converting esters and nitriles to aldehydes. The reaction is carried out at lower temperatures (typically -78 oC as that is the temperature of dry ice):

Remember that esters and nitriles are normally reduced by LiAlH4 to produce primary alcohols and primary amines respectively:

To understand why these reductions give different products, we need to remember that the reduction of carboxylic acids and their derivatives with LiAlH4 is a two-step addition of hydride ion to the carbonyl group. The first addition converts them to an aldehyde, and this, being more reactive than the precursor, is quickly reduced to an alcohol:

So, the question is how to stop the second addition and isolate the aldehyde as the final product.
From the mechanism of LiAlH4 reduction of esters to alcohols, we can see that the tetrahedral intermediate, formed after the first hydride intermediate, expels the alkoxy (RO–) component forming the intermediate aldehyde.

The idea behind using (or perhaps developing) the bulky reducing agent DIBAL is that first; it is not as electron-rich, thus not as powerful, and second; the bulky groups are additional factors preventing the second addition, or any type of possible involvement facilitating the expelling of the alkoxide ion.
Let’s now compare the mechanisms of LiAlH4 and DIBAL reduction of esters.
The Mechanism of DIBAL Reduction of Esters to Aldehydes
The reaction starts with the coordination of the oxygen to aluminum, which activates the carbonyl group by making the carbon more electron-deficient. After this, the hydride addition occurs, and a tetrahedral intermediate is formed, which, when kept at lower temperatures, does not expel the alkoxy component of the ester.
In the second part of the reaction, we add protic solvent such as an alcohol or water to quench the reaction and then hydrolyze the tetrahedral intermediate to the corresponding aldehyde under acidic conditions:
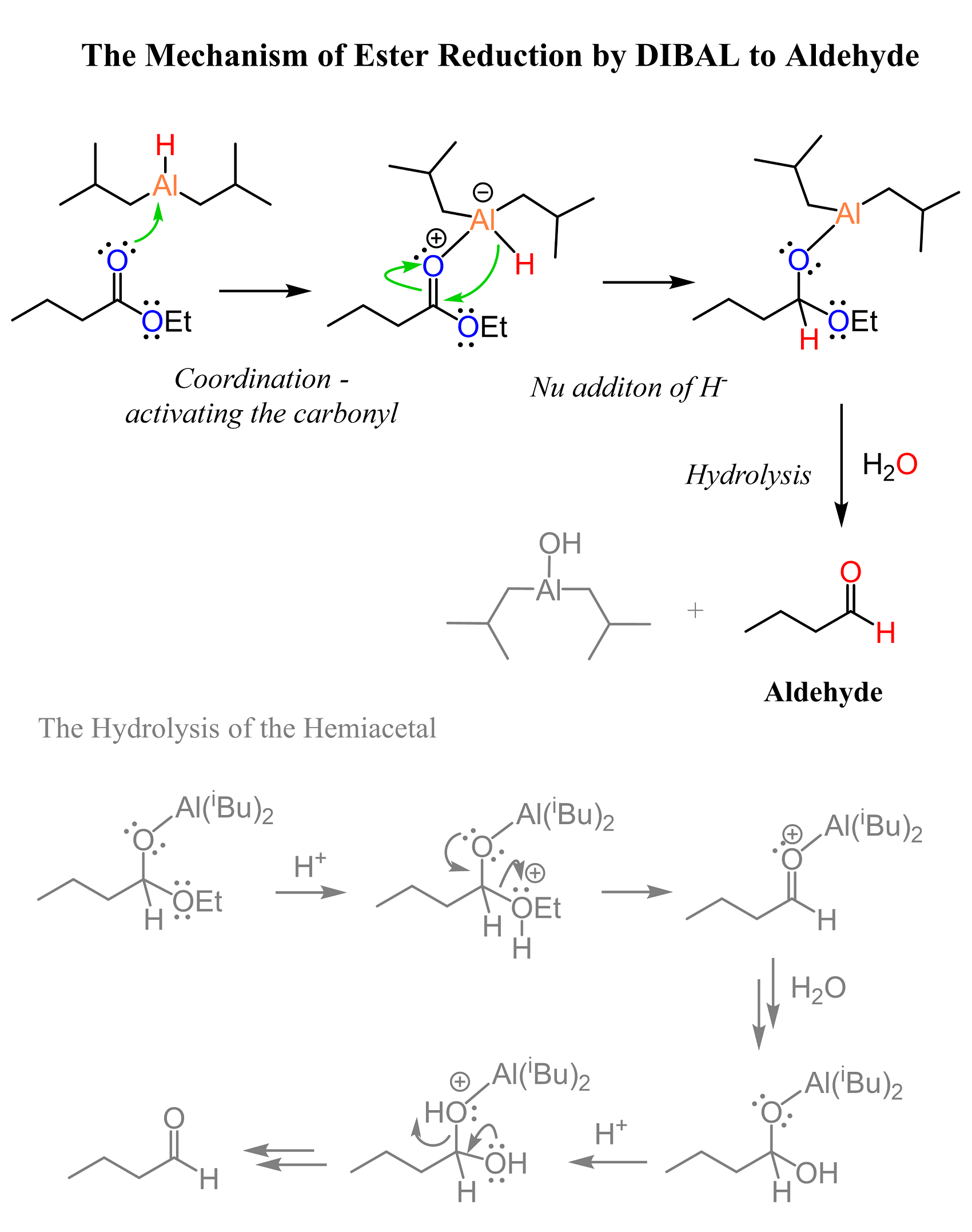
This mechanism is what we saw in the hydrolysis of hemiacetals, so feel free to check it for a better comparison.
Reduction of Nitriles
Like esters, nitriles can be reduced by LiAlH4 or DIBAL to primary amines and aldehydes respectively:
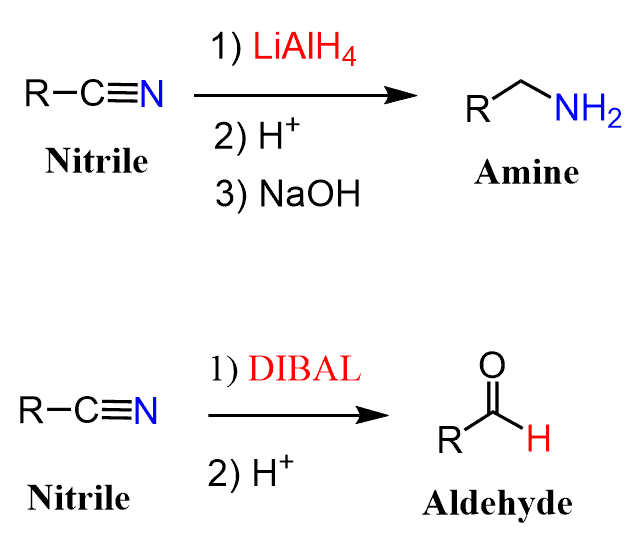
We have a separate post on the reduction of nitriles with LiAlH4, so feel free to check that out for the mechanism of the reaction.
The Mechanism of Nitrile Reduction by DIBAL
The reduction occurs by a coordination of the nitrogen to the aluminum thus activating the triple which allows for the addition of the hydride. Like in the reduction of esters to aldehydes using DIBAL, there is also a bulky intermediate formed after the addition of hydride to the unsaturated carbon of the nitrile. The bulkiness of the reducing agent and the iminium salt intermediate prevent the second hydride addition, and the salt is hydrolyzed to the corresponding aldehyde:

I will the hydrolysis of the iminium salt as an exercise to work on and you can use the example of ester reduction and the mechanism of hydrolyzing imines to aldehydes to do it.
At room temperature, DIBAL will reduce aldehydes, ketones, acids, and their derivatives to alcohols, so one thing about selective reduction of carboxylic acid derivatives to aldehydes is that they look a lot prettier on paper than in real life. Very often the reduction may go all the way to forming an alcohol, and sometimes, it is more practical to for the alcohol and oxidize it back to aldehyde.
Check Also
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
