Just like the aldol reaction, intramolecular Claisen reactions occur when five- or six-membered rings can be formed. These are called the Dieckmann condensation where one ester, of the same molecule, serves as an electrophile and the other is deprotonated and acts as a nucleophile. Here is the mechanism for a Dieckmann reaction forming a six-membered ring:
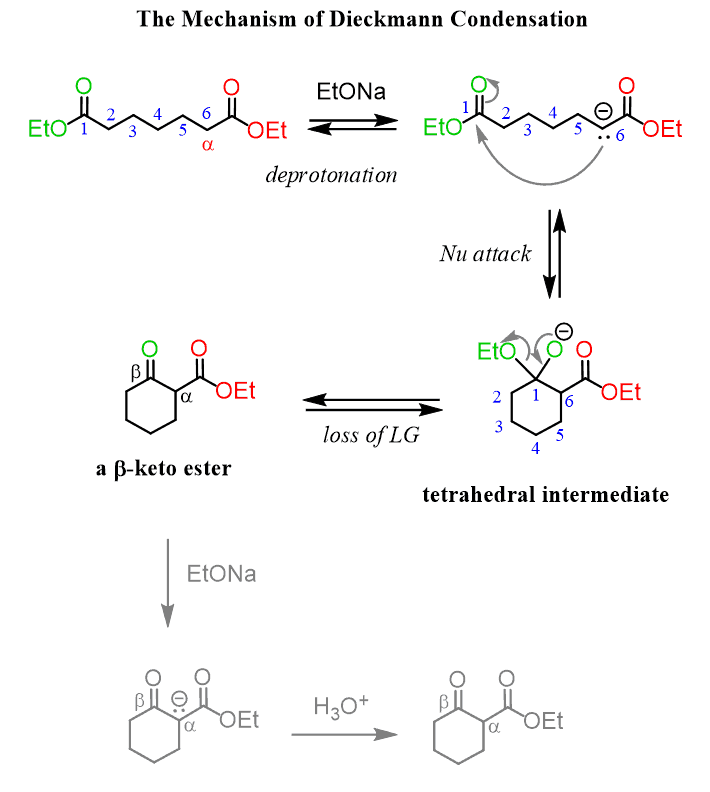
The product is a cyclic β-keto ester which has a very acidic alpha hydrogen. And it is this proton that makes any Claisen reaction possible since it is removed in an irreversible acid-base reaction which is the driving force that shifts the equilibrium in favor of the condensation.
This and the details for choosing a proper base for a Claisen and Dieckmann condensation are covered in this post.
Regiochemistry of Dieckmann condensation
Now, the example we discussed above is a symmetrical molecule and it wouldn’t matter which of the ɑ hydrogens is deprotonated. However, when this is not the case and there are two different ɑ positions in the molecule, a strong base can be used for selective enolate formation just like in the crossed Claisen and Aldol reactions:
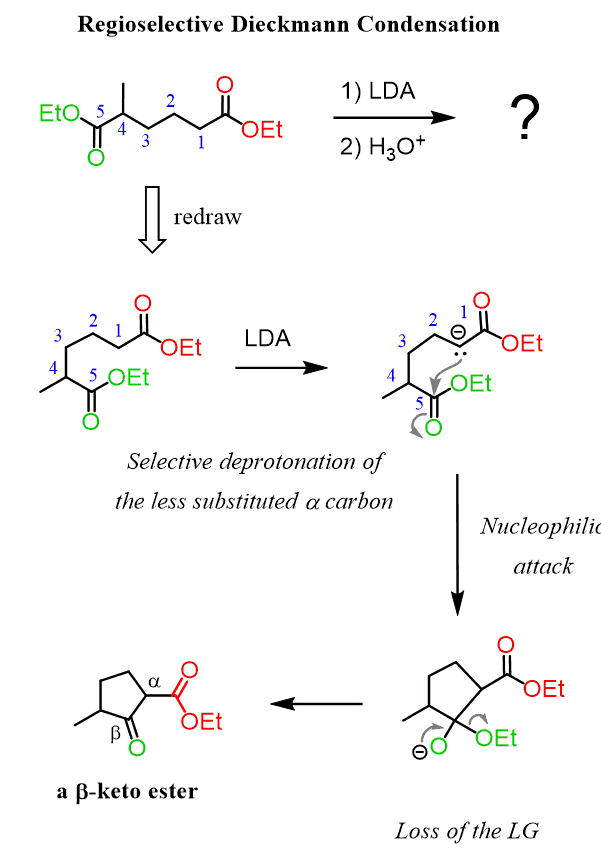
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
