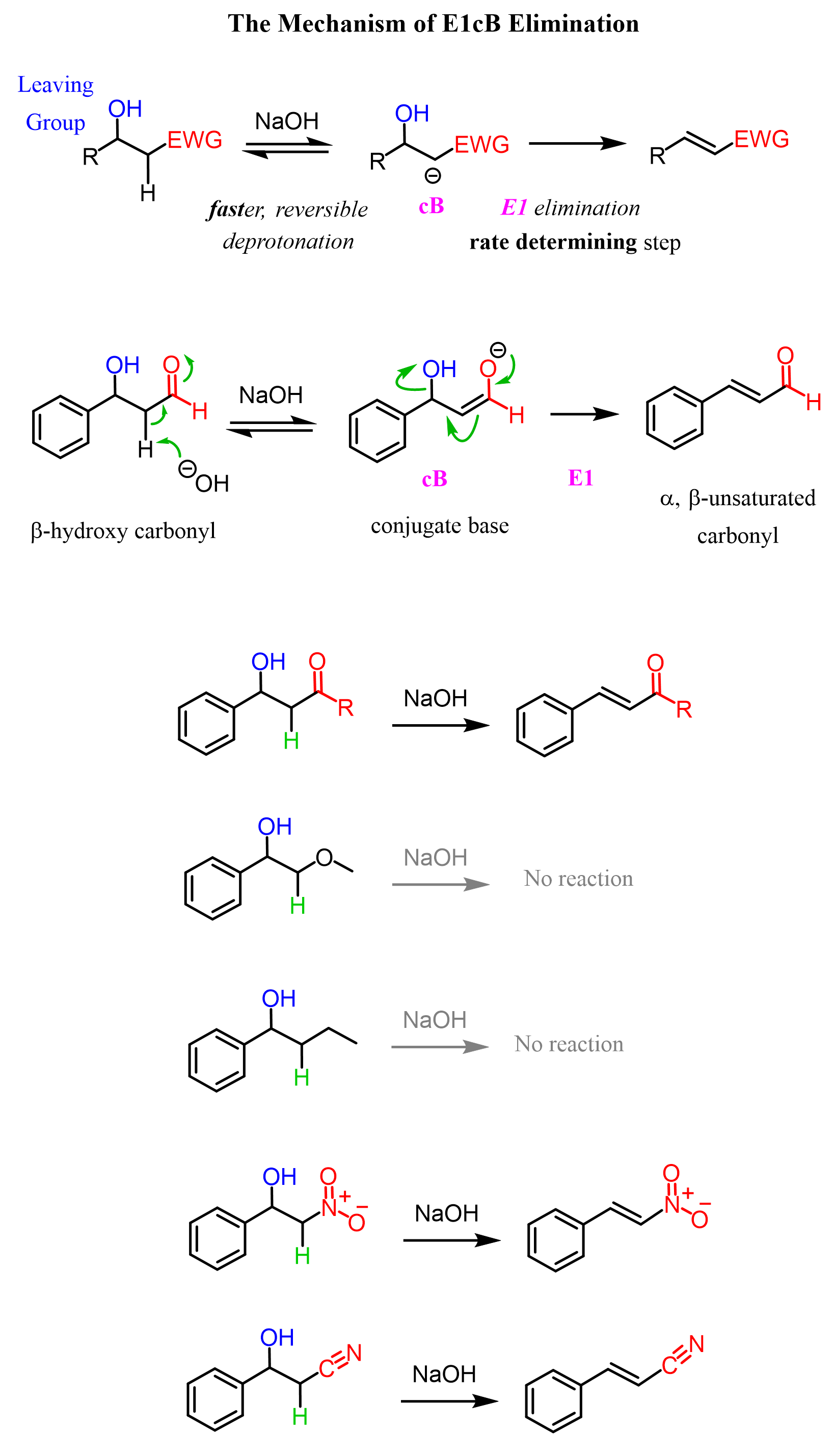
When a beta hydroxy carbonyl compound is treated with a moderately strong base, such as a hydroxide or an alkoxide, an elimination occurs by expelling the hydroxyl group. The most common example is the aldol condensation reaction:
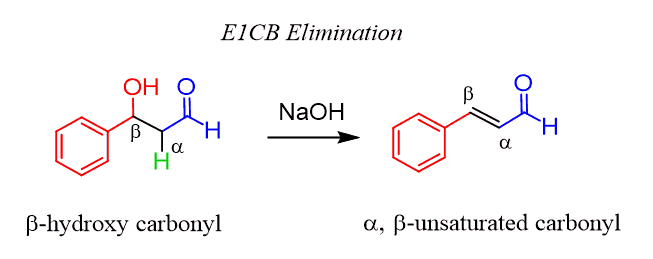
So, how does this happen? The name already suggests that it is an E1cB elimination, but let’s try to figure it out by relying on what we have learned about the elimination reactions. The most obvious, at least visually, way would be to attack the proton with the base and perform an E2 elimination, expelling the OH group:
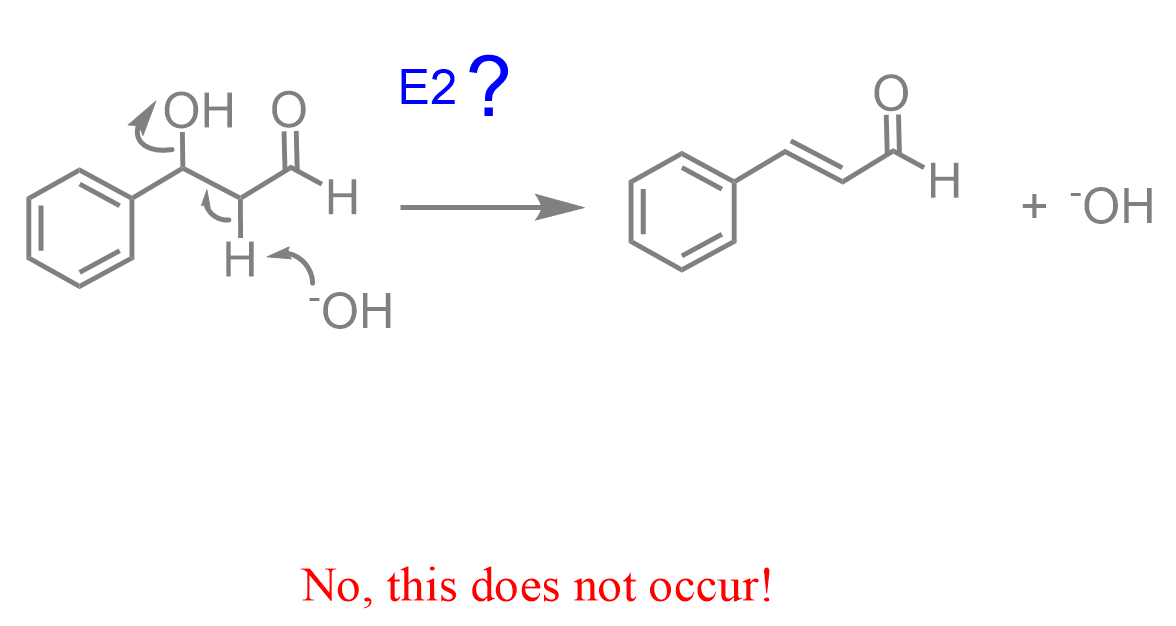
However, we know from the substitution and elimination reactions that OH– is a poor leaving group, or better to say, was never a leaving group in these reactions.
So, how else is the OH kicked out? Does the carbonyl have to do with this in any way?
It turns out that if we replace the aldehyde with another, non-resonance-withdrawing functional group, this reaction does not occur. For example, when the neighboring group of the highlighted hydrogen is a methoxy or an alkyl group, the elimination does not occur:
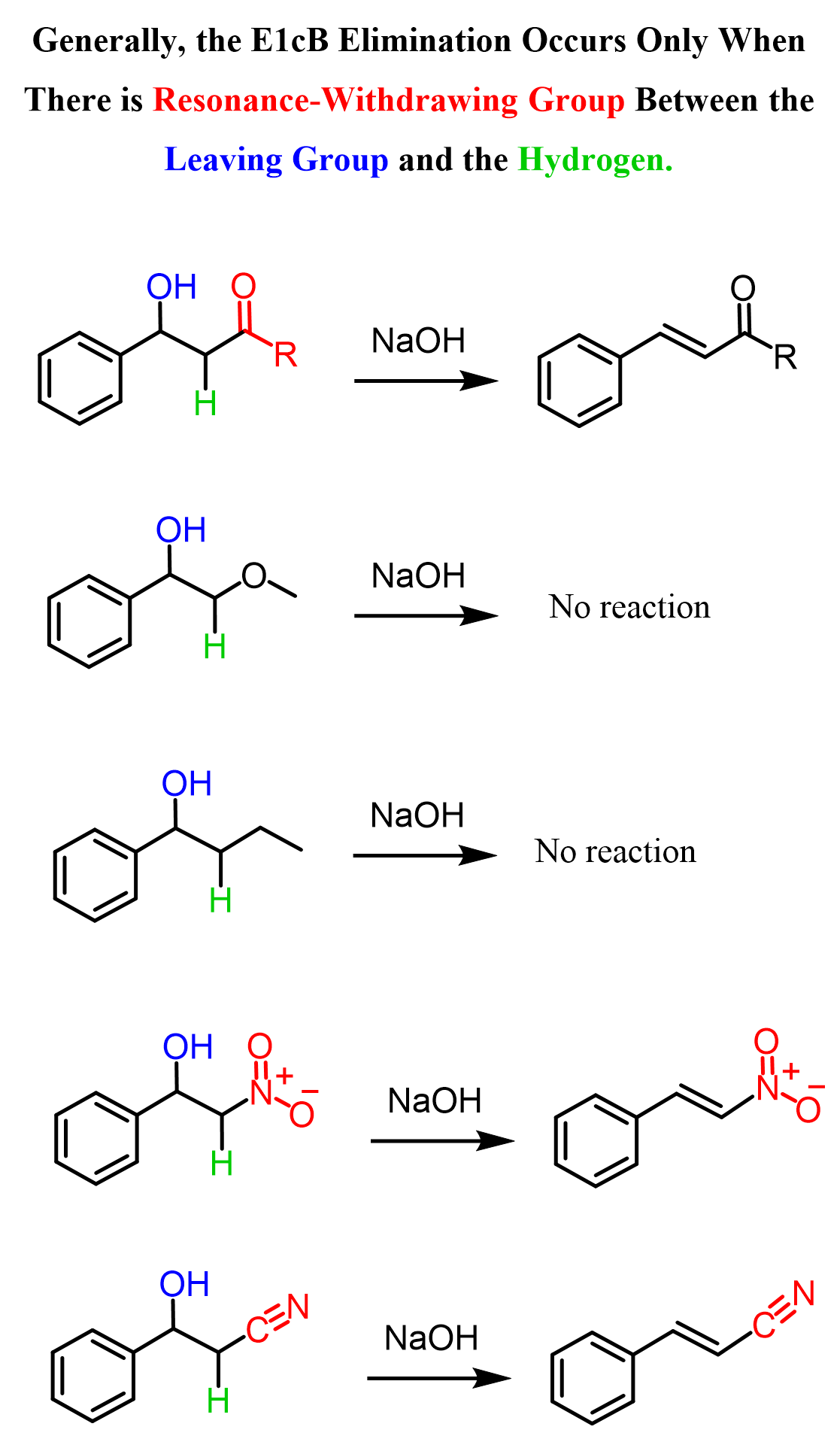
So, we need to figure out the role of the carbonyl group in this elimination to come up with a plausible mechanism. Now, you may recall from the aldol reaction itself, or from the keto-enol tautomerization, that a base such as OH–, even to a small extent, is capable of deprotonating the alpha hydrogen because of the resonance-stabilization of the resulting carbanion:
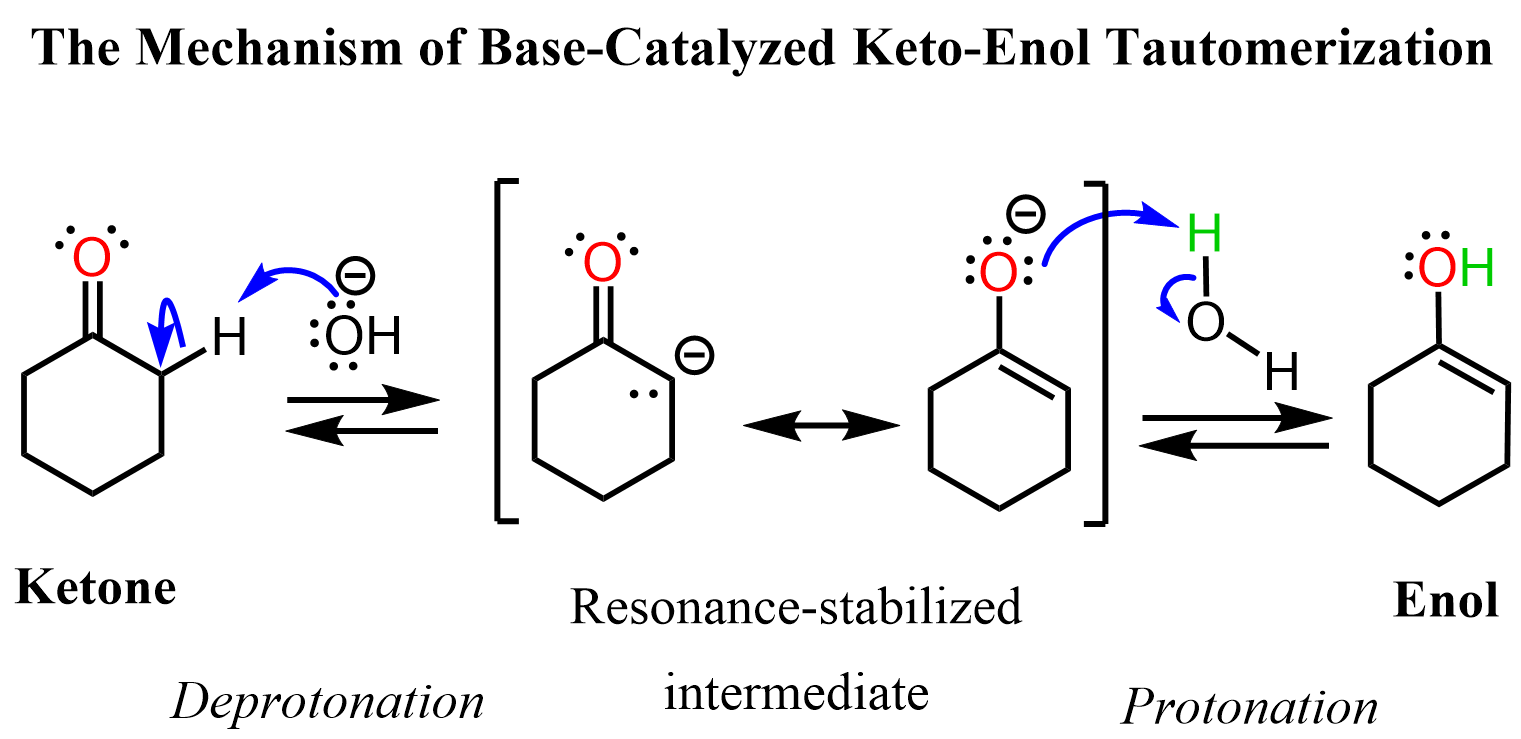
If this deprotonation didn’t occur, we wouldn’t have any of the condensation reactions we are (or already have) going to see in this chapter. The pKa of the alpha proton in ketones is about 20, whereas that of H2O (conjugate acid of OH–) is about 16, and therefore, the equilibrium of the deprotonation is shifted towards the ketone. The little amount of the enolate (resonance form in the brackets) reacts further, and this is what drags the reaction(s) forward.
So, we know that an enolate can be formed when the beta-hydroxy carbonyl is treated with NaOH. Let’s now see how this intermediate converts into an alpha-beta unsaturated carbonyl:
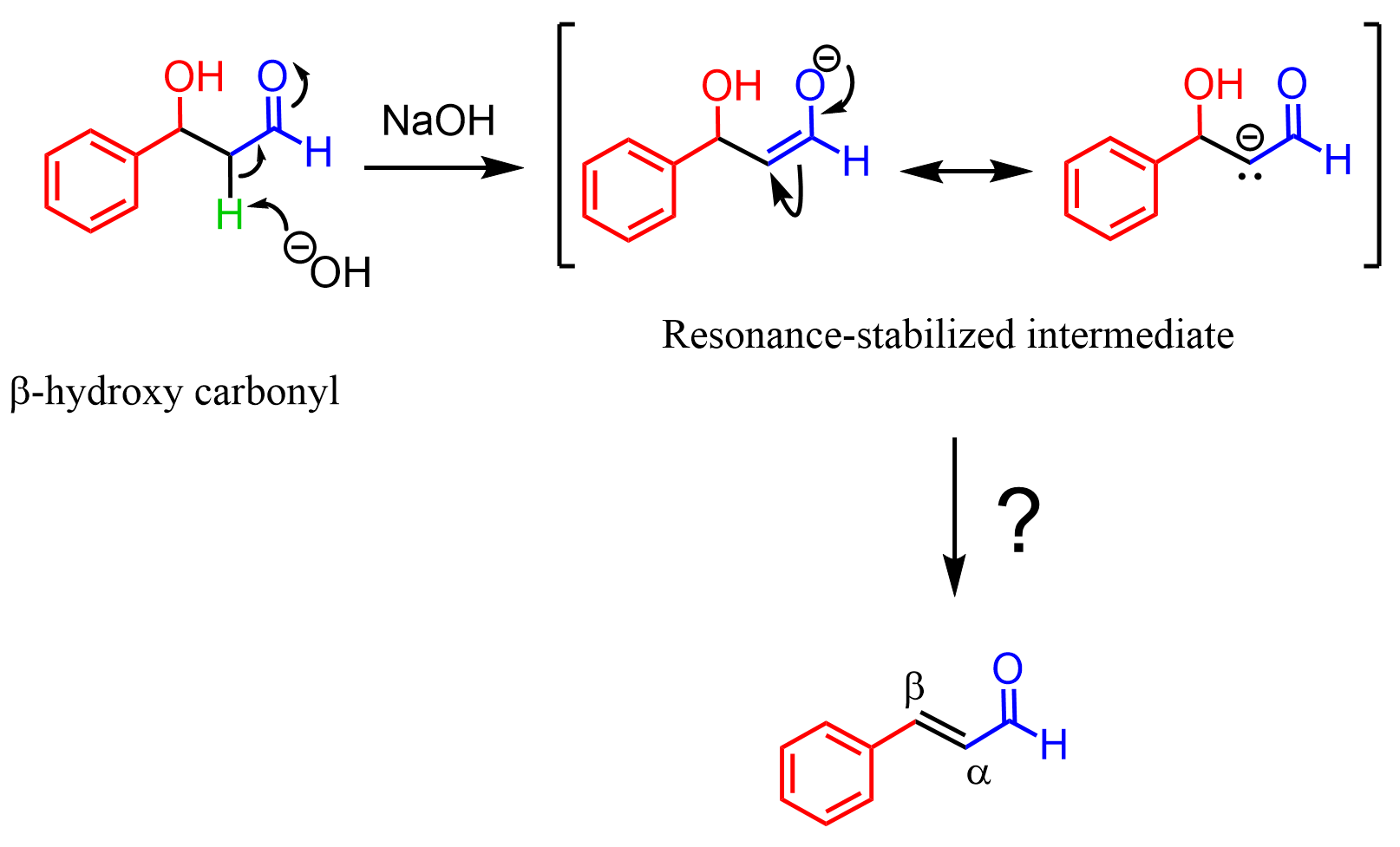
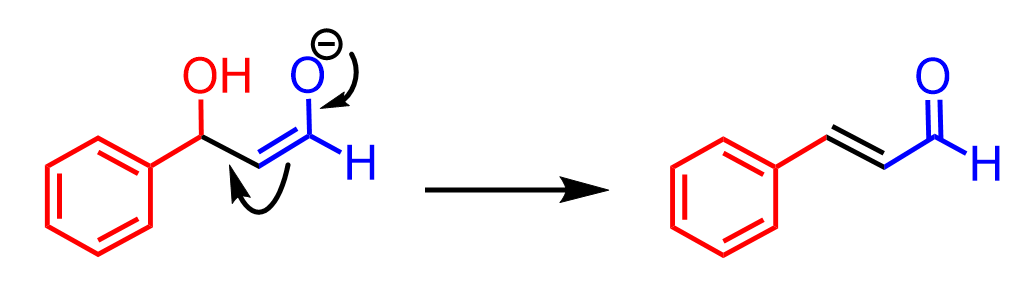
What happens is restoring the C=O carbonyl group, which pushes the π electrons to the carbon bearing the OH group. The newly forming C=C double bond then kicks out the OH- group, resulting in the final product α, β-unsaturated carbonyl. Notice that the final product is a conjugated system, and this is a driving force for this elimination:
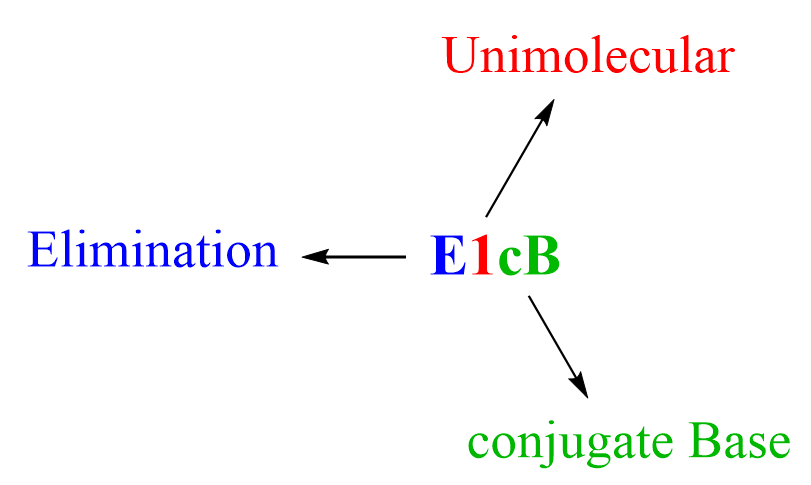
To summarize the steps of the E1cB mechanism, we can see that first, there is a deprotonation, which is possible because of the resonance-withdrawing group. This results in a carbanio,n which is the conjugate base of the substrate, and this conjugate base then undergoes elimination. Because it is the conjugate base undergoing elimination, thus the name E1cB (elimination, unimolecular, conjugate base):
Let’s now put all the steps together and show the mechanism for E1cB elimination with a general and specific example:
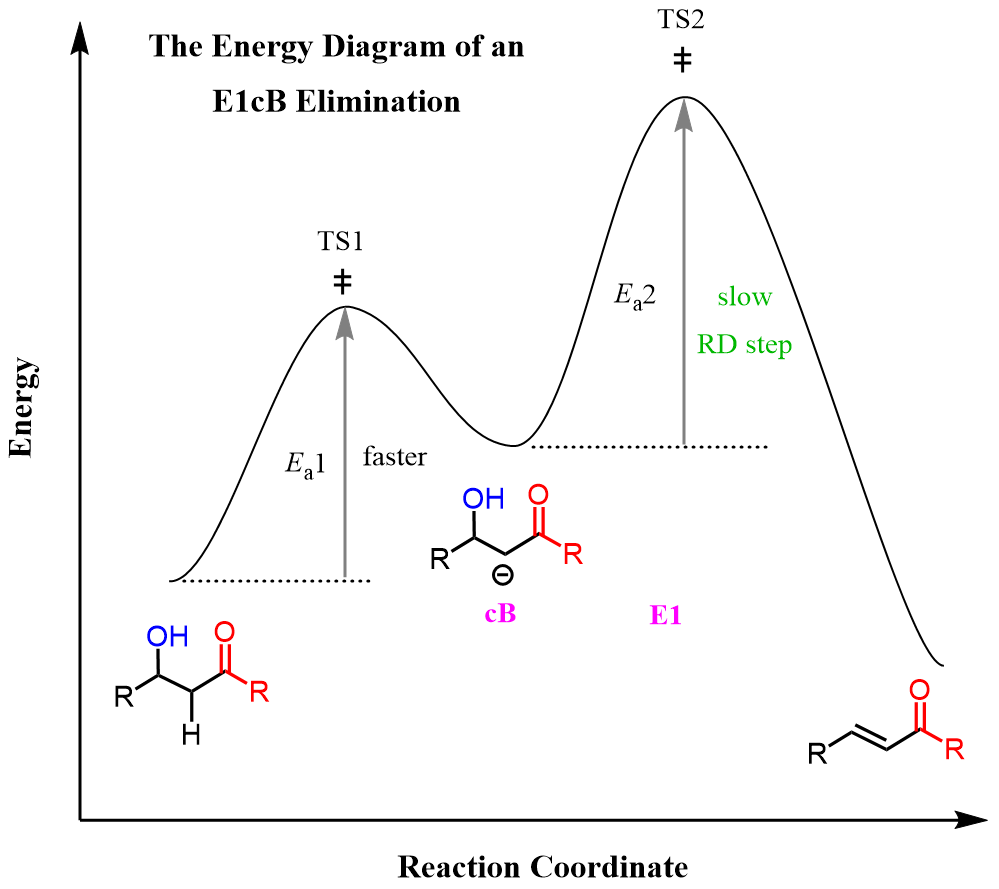
In most cases, the rate-determining step of the E1cB elimination reactions is the loss of the leaving group, so the general representation for the energy diagram of these reactions can be shown as:
Comparing E1cB with E1 and E2 Reactions
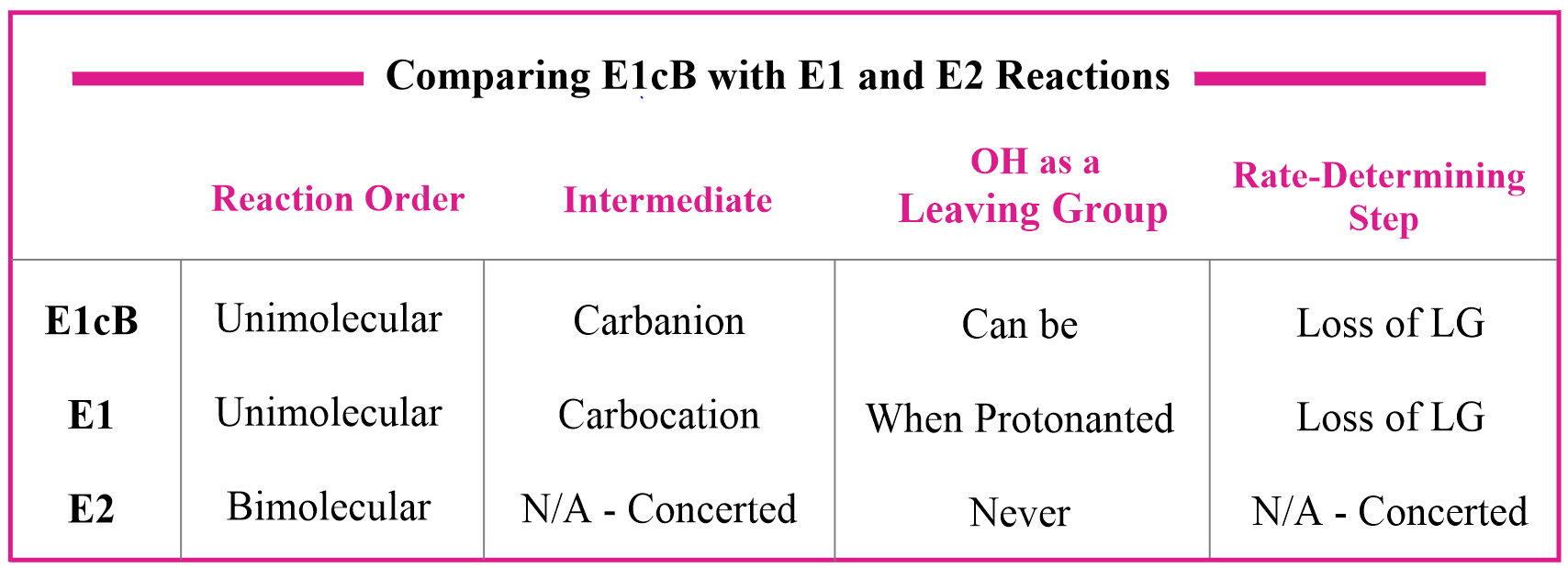
Let’s address some similarities of the E1cB mechanism with the E1 and E2 reactions. Both E1 and E1cB are unimolecular, which means the rate of the reaction depends on the concentration of the substrate and not the base. The difference here is that the intermediate in E1 elimination is a carbocation, whereas in E1cB it is a carbanion.

The difference with the E2 process is that the former is a bimolecular mechanism, so the rate depends on both the substrate and the base. Additionally, OH is never a leaving group in E2 reactions because it is a strong base and therefore, a poor leaving group.
So, how come it serves as a leaving group in E1cB reactions? One explanation for this is that in E2, it is lost from a neutral molecule, whereas in E1cB, it is lost from an anion, so it is not as energetically unfavorable:
E1cB Reactions with No Carbonyl and OH
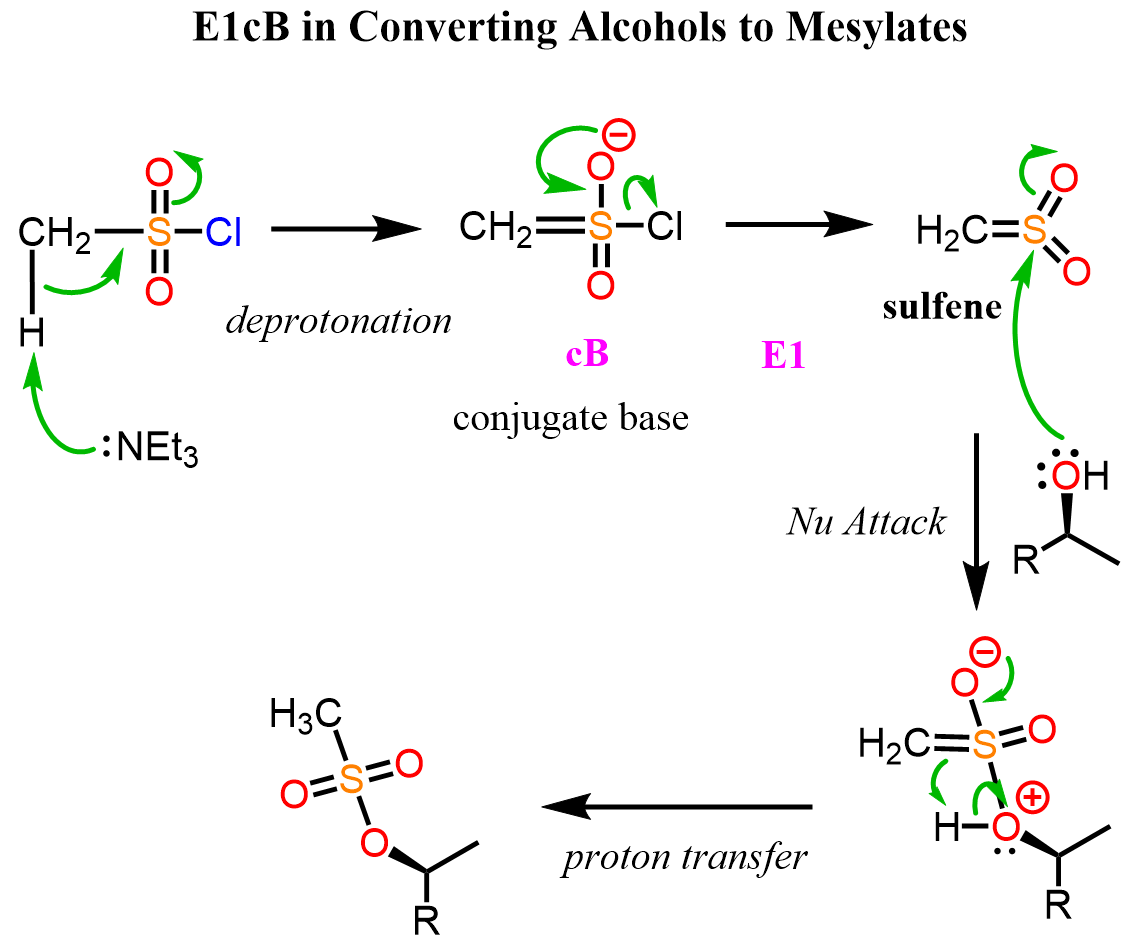
Although the E1cB elimination is most commonly encountered in carbonyl condensation reactions, we can still see it in other reactions. For example, earlier in Organic Chemistry 1, we learned about mesylates and tosylates, which are good leaving groups to involve alcohols and substitution and elimination reactions. During the mesylation of the hydroxyl group, an intermediate sulfene is formed from methanesulfonyl chloride via E1cB elimination:
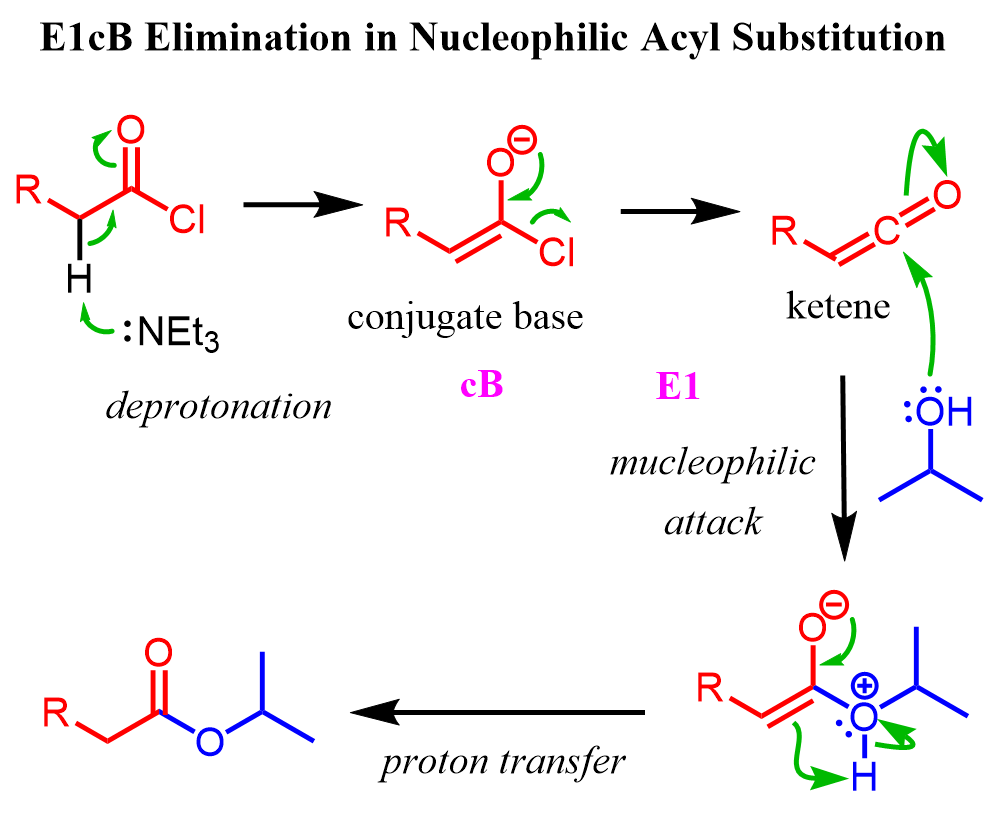
This is a general behavior for acid chlorides with alpha hydrogens (acidic protons next to the carbonyl group). Whenever treated with a (amine) base, they tend to undergo an E1cB, forming a highly reactive intermediate keten,e which is then attacked by the nucleophile:
Recall that, unlike the mesylation, the nucleophilic attack of the alcohols occurs directly on the sulfur during tosylation because toluene sulfonyl chloride lacks an acidic proton.
Another example is the formation of the benzyne intermediate in nucleophilic aromatic substitutions. In the first step, the benzene derivative bearing an electron-withdrawing group is deprotonated by a strong base, sodium amide, forming an anionic intermediate, and in the second step, this conjugate base undergoes elimination, forming the ring with a triple bond. The benzyne intermediate is then attacked by the –NH2 nucleophile:
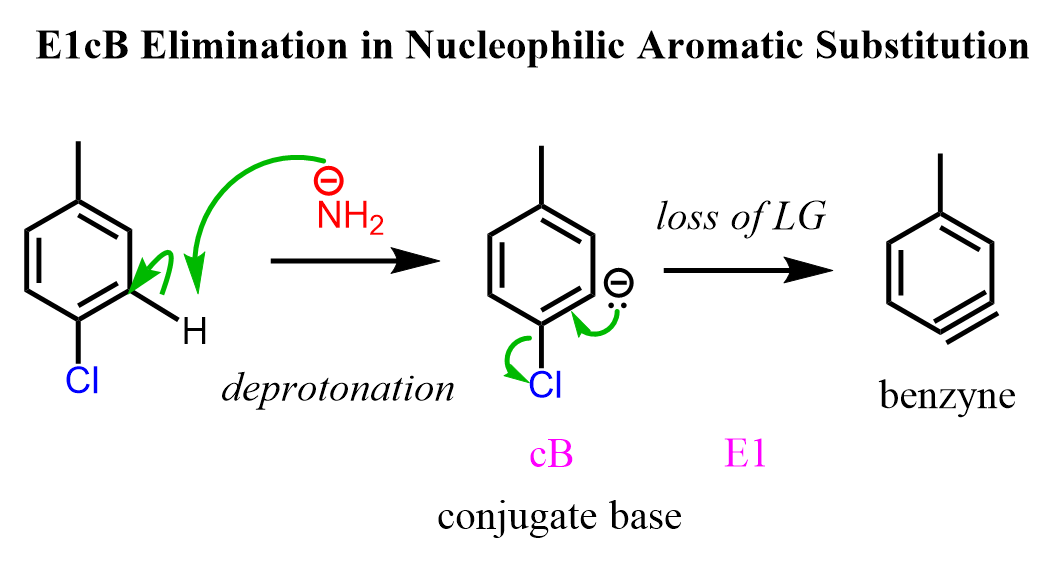
The rate-determining step of the reaction depends on the halogen. According to March’s Advanced Chemistry, when the leaving group is Br or I, proton removal is rate-determining, and the rate order for this step is F > Cl > Br > I. When Cl or F is the leaving group, breaking the C-X bond, or the formation of the benzyne intermediate, is rate-determining, and
the order for this step is I > Br > Cl > F. Notice that regardless of the halogen, deprotonation of the aromatic ring as the resulting carbanion is stabilized by the inductive effect of the halogen rather than a resonance stabilization.
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- The Cannizzaro reaction
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Decarboxylation
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann Condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Mannich Reaction
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
